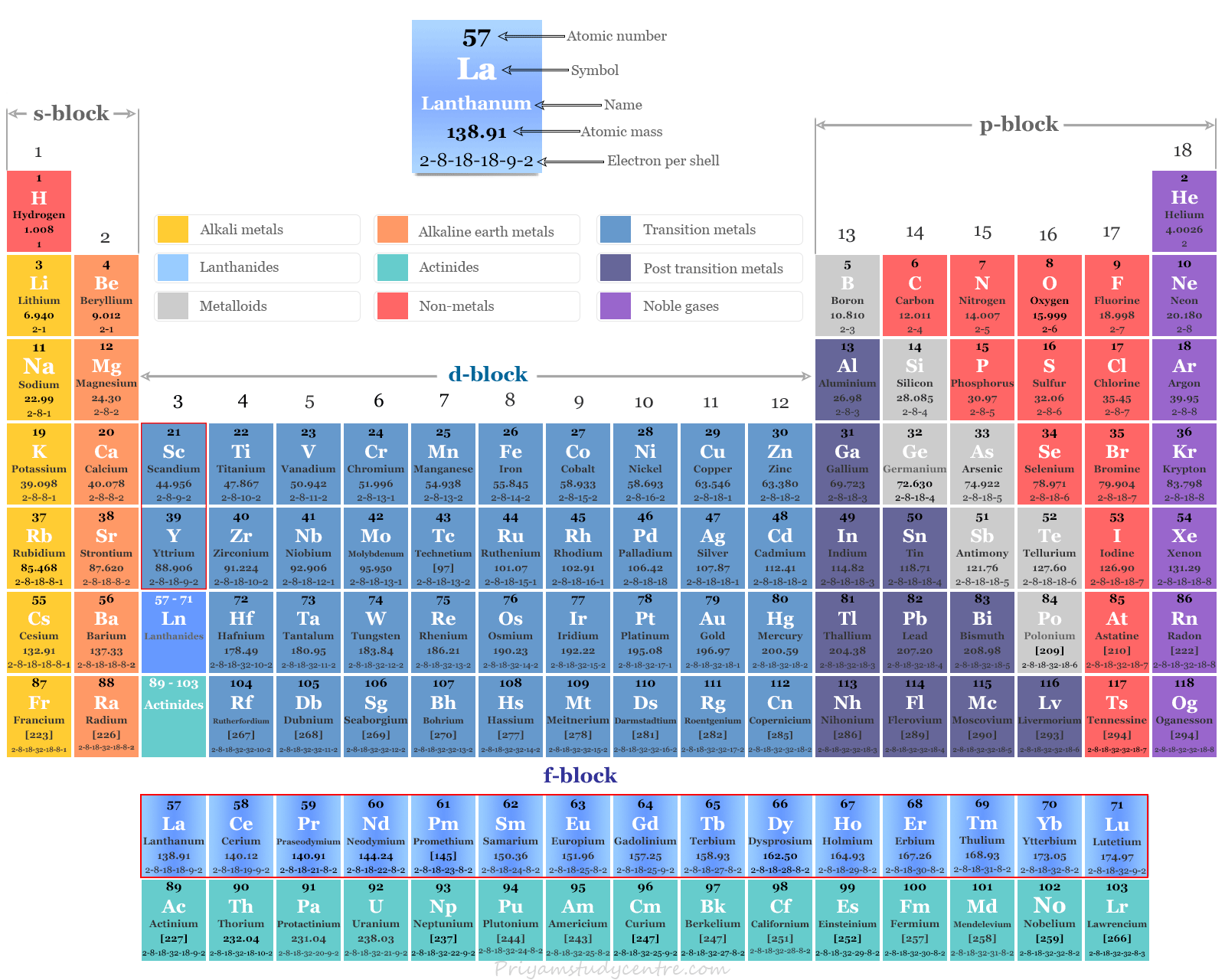
Periodic Table of Elements Rare Earth Metals
Rare earth elements or rare earth metals also called lanthanides or inner transition metals are the chemical elements from cerium (Ce) to lutetium (Lu) or f-block elements of the periodic table. Lanthanum is part of d-block elements according to their outer electronic configuration (5d1 6s2). The physical and chemical properties and common oxidation number or state are similar to that of the lanthanides series. Therefore, lanthanum is part of the lanthanides series. The seventeen elements (fifteen lanthanides and two elements like scandium and yttrium) together are called rare earth elements. All the elements from lanthanum to lutetium are members of the lanthanides series. But the seventeen elements from lanthanum to lutetium, scandium, and yttrium are members of the rare earth family.
Why Lanthanides are Called Rare Earth Elements?
The name lanthanides is given rare earth elements because they are extracted from oxides for which the ancient name is earth.
The elemental oxides of these metals are considered to be rare. The term rare earth elements is avoided now because many of these elements are no longer rare but abundant.
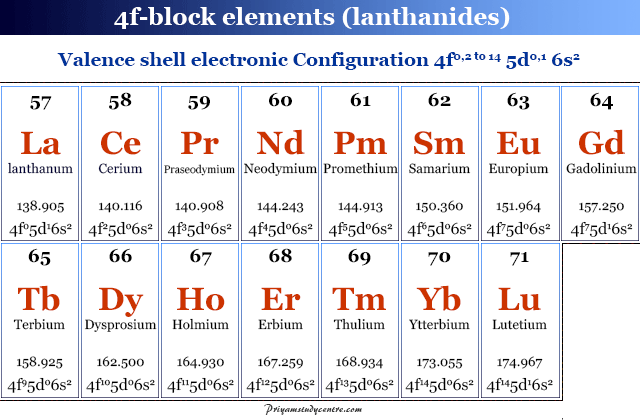
Electronic Configuration of Rare Earth Metals
The general electronic configuration of lanthanides is 4f0, 2 to 14 5d0,1 6s2. The elements scandium and yttrium are considered to be d-block elements. However, due to chemical properties and occurrence, these two elements are also members of the rare earth family.
| Rare earth element electronic configuration | |||
| Elements | Symbol | Atomic number | Electronic configuration |
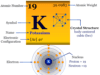
| Scandium | Sc | 21 | [Ar] 3d1 4s2 |
| Yttrium | Y | 39 | [Kr] 4d1 5s2 |
| Lanthanum | La | 57 | [Xe] 4f0 5d1 6s2 |
| Cerium | Ce | 58 | [Xe] 4f1 5d1 6s2 |
| Praseodymium | Pr | 59 | [Xe] 4f3 5d0 6s2 |
| Neodymium | Nd | 60 | [Xe] 4f4 5d0 6s2 |
| Promethium | Pm | 61 | [Xe] 4f5 5d0 6s2 |
| Samarium | Sm | 62 | [Xe] 4f6 5d0 6s2 |
| Europium | Eu | 63 | [Xe] 4f7 5d0 6s2 |
| Gadolinium | Gd | 64 | [Xe] 4f7 5d1 6s2 |
| Terbium | Tb | 65 | [Xe] 4f9 5d0 6s2 |
| Dysprosium | Dy | 66 | [Xe] 4f10 5d0 6s2 |
| Holmium | Ho | 67 | [Xe] 4f11 5d0 6s2 |
| Erbium | Er | 68 | [Xe] 4f12 5d0 6s2 |
| Thulium | Tm | 69 | [Xe] 4f13 5d0 6s2 |
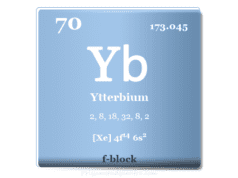
| Ytterbium | Yb | 70 | [Xe] 4f14 5d0 6s2 |
| Lutetium | Lu | 71 | [Xe] 4f14 5d1 6s2 |
Properties of Rare Earth Elements
- All the rare earth metals are silvery-white, soft, and malleable with high electrical conductance. Europium and ytterbium are a pale yellow colour.
- They are highly electropositive and reactive and the heavier elements are comparable to calcium and aluminum.
- The compact metals are quite stable to dry air at ordinary temperatures. In a humid atmosphere, they turn rapidly into dull.
- All of the rare earth elements burn in the air to form oxides and nitrides with oxygen and nitrogen.
Oxidation State of Rare Earth Elements
The stable and common oxidation state of rare earth metals is +3 (III). It is possible to correlate the stability of lanthanides in various oxidation states with the electronic configuration of their ions.
On the basis of the general rule, half-filled and completely filled 4f orbitals are highly stable. Therefore, Ce+4, La+3 (4f0), Tb+4, Eu+2, Gd+2 (4f7) and Yb+2, Lu+3 (4f14) ions are stable. The charge of the respective ions is equal to its oxidation state or number.
Magnetic Properties of Rare Earth Elements
The paramagnetic property of an ion or an atom defines the presence of an unpaired electron on it. Since both the rare earth metals ions like La+3 (4f0 5d0 6s0) and Lu+3 (4f14 5d0 6s0) have no unpaired electrons. Therefore, these two rare-earth ions have diamagnetic properties. Other ions rare-earth elements like Ln+3 (Ln = lanthanides) show paramagnetic properties.
Lanthanide Contraction
Except for scandium and yttrium, the atomic and ionic radii of rare earth metals steadily decrease along with the lanthanide series. This is commonly known as lanthanide construction.
Poor shielding electrons of 4f-orbitals steadily raise the effective nuclear change along with the series. Due to this fact, the atomic and ionic radii of lanthanides decrease across the series.
Where are Rare Earth Metals Found?
There are more than 200 minerals known which contain rare-earth elements. The two most commercially important minerals of rare earth are monazite and bastnaesite.
- Monazite is a mixed phosphate of lanthanum, cerium, thorium, and other rare earth metals.
- While bastnaesite is a fluoride carbonate of lanthanides and other rare earth metals heavier rare earth elements are virtually absent from it.
- Xenotime is also another rare-earth orthophosphate containing thorium and a high percentage of yttrium.
Monazite is a chemically quite inert rare earth mineral. Such rare earth mineral is found mostly in high density in beach sands and river beds through weathering. The rare earth mineral monazite is found mainly in India, China, South Africa, Australia, and Malaysia.
Why are Rare Earth Metals Important?
All rare earth metals are very important elements because they are used widely in different chemical processes and chemical industries. Lanthanides or rare-earth metals are used in metallothermic reactions due to their extraordinary reduction character. The process used for the production of pure niobium, zirconium, iron, cobalt, nickel, manganese, tungsten, uranium, boron, and silicon.
Mischmetal
Alloys of lanthanides are known as mischmetals. The rare earth element, cerium (45 to 50 percent), lanthanum (25 percent), and neodymium (5 percent) are the major constituents of mischmetals.
Mischmetal is used for the production of different brands or types of steel with high corrosion resistance and workability. It is also an excellent scavenger for the adsorption of elements like oxygen and sulfur. Rare earth alloys are used for making permanent magnets. Neodymium is particularly used in making magnetic alloys.
Uses of Rare Earth Elements
The uses of rare-earth compounds can be broadly classified into two types, non-nuclear applications, and nuclear applications.
Rare Earth Oxides Ceramics
For decolorizing glasses, we used oxides of rare earth elements like cerium, lanthanum, neodymium, and praseodymium. These elements are used for the production of protective transparent glass blocks. The blocks are used mostly in nuclear technology to protect against radioactive radiation coming from nuclear reactions.
The oxides of rare earth metals absorbed ultraviolet rays from sunlight. Therefore, the glasses that contain lanthanum oxide are used for the production of sunglasses, and goggles for glass blowing, and welding work.
Paints and Textiles Industries
The rare earth compounds are used for the production of lakes, dyes, and paints for porcelain. Ceric salts are used for dying In textile industries, we used ceric salts for drying clothes. Chloride and acetate of rare earth metals are also used for making fabrics that are water-proof and acid-resistant.
Catalytic Applications
Certain compounds of lanthanides are also used as a chemical catalyst for hydrogenation, dehydrogenation, and oxidation of various organic compounds. For example, cerium phosphate is a good chemical catalyst used for the production of petroleum.
Nuclear Applications
Lanthanide elements and their compounds are important materials in the nuclear power generation process. For example, rare earth elements like gadolinium, samarium, europium, and dysprosium have large cross-sections for neutron capture reactions and are used in control rods of atomic piles. Radioactive isotopes of praseodymium are used in solid oxide fuel cells.