Electron Spin Resonance (ESR) Spectroscopy
Electron spin resonance (ESR) also called electron paramagnetic resonance (EPR) spectroscopy is a method for studying paramagnetic substances that exhibit characteristic magnetic properties. The electron spin resonance phenomenon is shown by atoms having an odd number of electrons, ions having partially filled inner energy levels, and molecules that carry angular momentum. ESR spectroscopy is used mainly to study free radicals having unpaired electrons. These electrons remain after the homolytic fission of a covalent bond by ultraviolet or gamma radiation. ESR has limited application because it is observed primarily in systems that contain unpaired electrons. Therefore, the systems that can be investigated by ESR spectroscopy are organic and inorganic free radicals and ions of transition metals and rare earth metals that have unpaired d or f-electrons.
Electron spin resonance is another type of magnetic resonance that is connected with the magnetic behavior of spinning electrons. Both NMR and ESR spectroscopy collectively show magnetic resonance phenomena. But NMR is observed in the radiofrequency region and ESR is observed in the microwave region of the electromagnetic spectrum. The theoretical principles of ESR and NMR are similar.
Principle of ESR Spectroscopy
In electron paramagnetic resonance (EPR), the energy levels are produced by the interaction of the magnetic moment of an unpaired electron in a molecule with an applied magnetic field. The ESR spectrum is obtained due to the transition of these energy levels by absorbing radiations with microwave frequency.
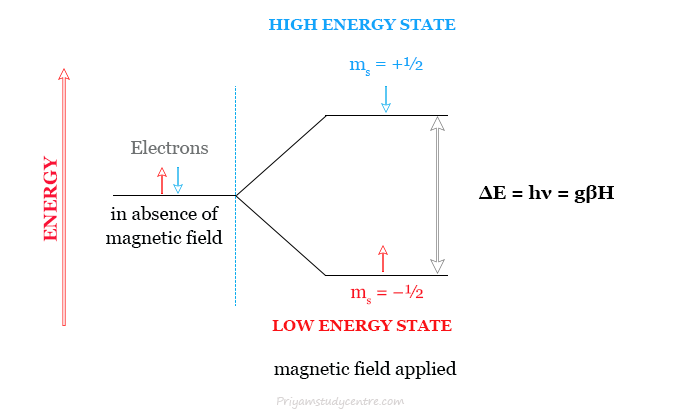
For an electron, the spin angular momentum quantum number (ms) will have values of ±½. In the absence of a magnetic field, the two values of ms will give rise to a doubly degenerate spin energy state. If a strong magnetic field H is applied the degeneracy is removed to give two non-degenerate energy levels.
- The low-energy state will have the spin magnetic moment aligned with the field and corresponds to the quantum number, ms = −½.
- The high-energy state will have the spin magnetic moment opposed to the field and corresponds to the quantum number, ms = +½.
For electron spins that are parallel in a field, E = μH. Whereas, for those which are aligned antiparallel, E = −μH. Here, μ = magnetic moment of the spinning electron.
Electron spin resonance (ESR) spectrum
In the ESR spectrum, a transition between the two different energy levels takes place by absorbing a quantum of radiation of frequency in the microwave region. Therefore, the ESR spectrum of a free electron would consist of a single peak.
When the absorption occurs, the following relation holds good for the ESR spectrum,
2μH = hν
For a free electron, the energy (ΔE) of transition is more accurately represented by the following relation,
ΔE = hν = gβH
where h = Planck’s constant,
ν = frequency of microwave radiation,
H = applied magnetic field,
β = Bohr’s magneton = eh/4πmc = 0.9723 × 10−20 erg/gauss,
g = spectroscopic splitting factor or Lande’s splitting factor. For a free electron g = 2.0023 but this value is slightly modified for electrons in molecules.
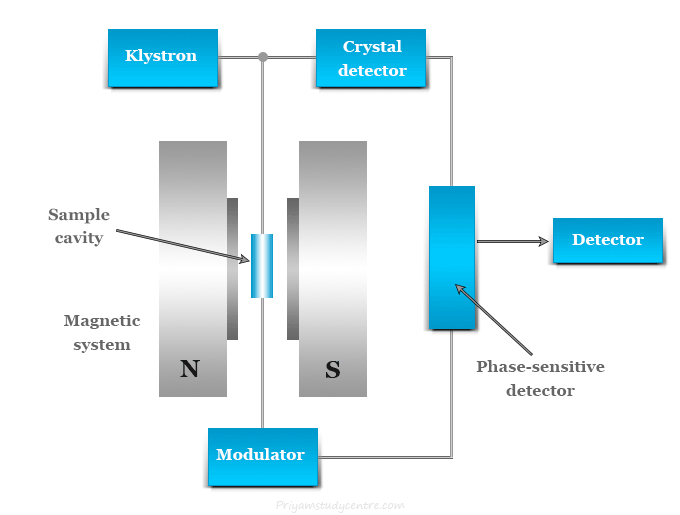
Electron Spin Resonance Spectrometer
The energies required to bring about a transition are different in ESR and NMR. In ESR, transitions occur at frequencies in the microwave region whereas, in NMR, transitions occur in the radiofrequency regions.
A typical ESR spectrometer contains the following main components,
- Source (Klystron, isolator, wavemeter, and attenuator)
- Circulator or magnetic T
- Sample cavity
- Magnetic system
- Crystal detectors
- Autoampilfier and phase-sensitive detector
- Oscilloscope or Pen Recorder
A source in electron spin resonance
In an ESR spectrometer, the various components of a source may include:
- Klystron: A klystron is a vacuum tube that can produce microwave oscillations centered on a small range of frequencies. In most of the ESR spectrometers, the source is the klystron oscillator operating in a microwave region with a wavelength of 3 cm.
- Isolator: It is a non-reciprocal device that minimizes microwave vibration produced by oscillators.
- Wavemeter: The wavemeter is put in between the isolator and the attenuator. It is used to know the frequency of the microwave produced by the klystron oscillator.
- Attenuator: Between the wavemeter and circulator, there is an attenuator that adjusts the level of the microwave power falling upon the sample.
Circulator or Magic T
The microwave radiations obtained from the klystron enter the circulator through a waveguide by a loop of wire which is coupled with an oscillating magnetic field. It sets up a corresponding field in the waveguide.
A waveguide is generally made up of a hallo rectangular copper or brass tubing. It has silver or gold plating inside the tube for the production of a highly conducting flat surface.
The operation of the four-port circulator in ESR spectroscopy is given below in the picture,
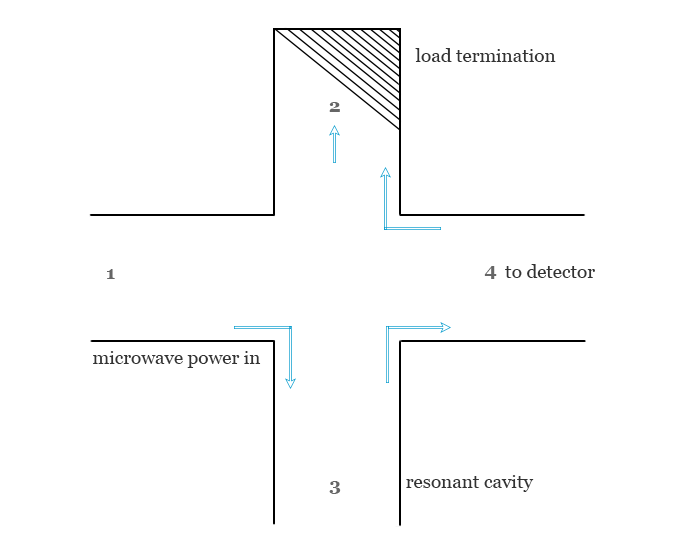
- The microwave radiation enters arm 1.
- Arm 2 is connected to the resonant cavity and sample.
- Generally, arm 4 contains a terminating load for absorbing any power that might be reflected from the detector arm.
- Arm 4 is attached to the detector of the electron spin resonance spectrometer.
Sample cavity
The sample cavity is an important component of the electron spin resonance (ESR) spectrometer. The cavity system is constructed in such a way that the applied magnetic field is maximum in the sample dimension.
In most ESR spectrometers, duel sample cavities are used due to simultaneous observation of a sample and a reference material. By use of reference material, the sources of error are compensated by comparing relative signal heights.
Magnetic system in ESR spectroscopy
In an EPR spectrometer, the resonant cavity is placed in a magnetic assembly that includes the magnet with a dedicated power supply. It has a homogeneous magnetic field that can vary from 0 to 500 gauss.
The magnetic field should be stable and uniform over the sample volume. The stability of the field can be enlarged by a high-regulating power supply system.
Crystal detectors in ESR spectroscopy
The most commonly used detector is a silicon crystal detector. In ESR spectrometers, it acts as a microwave rectifier that converts microwave power into a direct current (DC) output.
Autoampilfier and phase-sensitive detector
After detection by the crystal detector, the signal undergoes a narrow-band amplifier that amplifies the signal with a lot of noise. The noise can be reduced by the operation of the phase-sensitive detector.
Oscilloscope or Pen Recorder
Finally, the signal from the phase-sensitive detector and sweep unit is recorded by the oscilloscope or pen recorder.
- Dual-trace oscilloscopes with two vertical inputs are extremely useful components of electron spin resonance (ESR) instruments.
- In an analog oscilloscope, the vertical amplifier acquires the signals to be displayed. It provides a signal large enough to deflect the CRT’s beam and better delays the signal by a fraction of a microsecond.
- There are several inputs for voltages in most modern oscilloscopes. Therefore, it can be used to plot one varying voltage versus another.
- Some analog oscilloscopes have a feature of Z input. For example, if a pair of waves of known frequency is used to generate a circular Lissajous figure and a higher unknown frequency is applied to the Z input.
Application of Electron Spin Resonance (ESR) Spectroscopy
Electron spin resonance (ESR) or electron paramagnetic resonance (ESR) spectroscopy is used in various fields of science such as analytical chemistry, biochemistry, and physics for the detection and identification of free radicals in solids, liquids, or gases. It is also used for the detection and identification of paramagnetic centers such as F-centers.
Application in biochemistry
Electron spin resonance (ESR) spectroscopy has been widely applied in biochemistry for quantitative and qualitative analyses of reactive oxygen species (ROS) and reactive nitrogen species (RNS).
The ESR spin-trapping method was developed in the early 1970s and used for the analysis of short-lived free radicals. It is now widely used or one of the most powerful tools for free radical analysis. Some interesting examples of biological analysis are,
- The presence of free radicals studied by electron spin resonance spectroscopy (ESR) is useful for the detection and identification of healthy and diseased tissues.
- Most of the oxidative enzymes function via one-electron redox reactions involving the production of enzyme-bound free radicals or by a change in the valence state of transition metal ions. It may be confirmed by ESR studies.
- The ESR studies of various biological systems such as leaves, seeds, and tissues correlate between the concentration of free radicals and the metabolic activity of the materials.
- Photosynthesis is a process where plants convert solar energy obtained from sunlight into chemical energy. ESR is used to study photosynthesis carried out by photosynthetic bacteria.
Application in analytical chemistry
Direct applications of electron spin resonance spectroscopy are being used to determine various substances.
- ESR spectrum used to determine Mn+2 ions in solution. It shows six lines. The multiplicity = 2l + 1, where I = 5/2.
- ESR or EPR spectrum provides a rapid and convenient method for the determination of vanadium in petroleum products.
- Electron spin resonance spectroscopy is also used to estimate copper (II), chromium (III), gadolinium (III), iron (III), and titanium (III).
- The ESR spectrum may be used to estimate polynuclear hydrocarbons which are first converted into radical cations and then absorbed in the surface of an activated silica-alumina catalyst.
Miscellaneous applications
- The oxidation state of a metal ion can be determined by the use of the ESR spectrum. For example, the ESR spectrum of the Cu (I) complex gives a much-reduced signal than the Cu (II) complex. The difference between the signals of the two oxidation states of copper may be used to determine the state of copper in many complexes and biological compounds.
- Electron spin resonance spectroscopy is used to confirm the impurities of semiconducting materials that are responsible for the conducting properties of semiconductors.
- It is successfully investigating the complexes of copper, nickel, cobalt, and sodium with maleonitriledithiolate.
- ESR has been applied to the study of chemical, photochemical, and electrochemical reactions proceed via the free radical mechanism.
- Electron spin resonance or ESR spectroscopy may be used to detect the conduction of electrons in solutions of alkali metals in liquid ammonia, alkaline earth metals, alloys, etc.