Niobium Element
Niobium is a chemical element or light grey, crystalline, transition metal of Group 5 (VB) of the periodic table with symbol Nb and atomic number 41. It is used mainly for making stainless steel and other alloys. In the year 1801, English chemist Charles Hatchett identified the new element niobium in the mineral from North America and named it columbium which comes from the mineral columbite. The element columbium discovered by Hatchett was a mixture of the new element tantalum. Both niobium and tantalum are silvery-white bright metals with very high melting points. They are from the body-centered cubic crystal lattice. The effect of lanthanide construction makes the chemical properties like atomic and ionic radii of the two metals very similar. Therefore, the chemistry of Nb and Ta is very similar but the electronic distribution is different.

Where is Niobium Found?
Niobium is found in the earth’s crust to the extent of 20 ppm. Owing to the great chemical similarities with tantalum, it always occurs with tantalum.
The main mineral of the metal is columbite or tantalate with the chemical composition (Fe, Mn)M2O6, M = Nb, Ta. Therefore, it is obtained as a byproduct of the extraction of tin in Malaysia and Nigeria.
Properties of Niobium
They are malleable and ductile in a pure state. However, the high melting and boiling points of the metal indicate the participation of a large number of d-electrons in metallic bonding. It is oxidized on heating in air and dissolved in fused alkali.
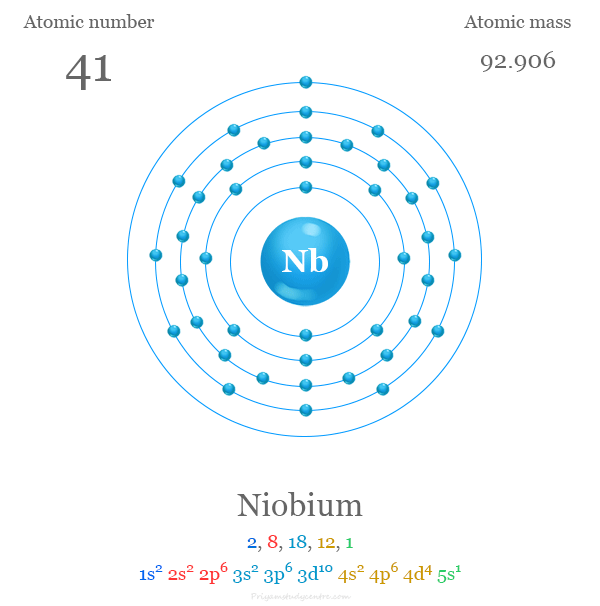
Electron Configuration of Niobium
The 41 electrons of the niobium atom are distributed in different energy levels to show the following electronic configuration given in the picture,
| Properties of Niobium |
|||
| Atomic number | 41 | ||
| Electron per shell | 2, 8, 18, 12, 1 | ||
| Electronic configuration | [Kr] 4d4 5s1 | ||
| Block | d-block | ||
| Period | period 5 | ||
| Group | group 5 | ||
| Atomic weight | 92.906 | ||
| Melting point | 2750 K (2477 °C, 4491 °F) | ||
| Boiling point | 5017 K (4744 °C, 8571 °F) | ||
| Density | 8.57 g/cm3 | ||
| Molar heat capacity | 24.60 J mol−1K−1 | ||
| Electrical resistivity | 152 nΩ·m | ||
| Atomic radius | 146 pm | ||
| Covalent radius | 164±6 pm | ||
| Chemical properties | |||
| common oxidation number | +5 | ||
| Electronegativity | 1.60 (Pauling scale) | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 652.1 | 1380 | 2416 | |
Nb in the Periodic Table
It is placed in group 5 and period 5 of the periodic table. Niobium is a transition metal or d-block element that lies between zirconium and molybdenum.
Isotopes of Niobium
Niobium has one stable isotope 93Nb. 32 radioactive isotopes ranging from 81 to 113 have been obtained by different types of nuclear reactions.
The most stable isotopes like 92Nb have a radioactive half-life equal to 34.7 million years. The least stable isotope 113Nb has a half-life of 30 milliseconds.
Production Process
- The mineral of niobium and tantalum is fused with potassium carbonate and extracted mostly with water. The insoluble hydroxide of iron and manganese is largely eliminated by the process.
- The aqueous extract containing K3NbO4 and K3NbO4 is treated mostly with carbon dioxide to precipitate pentaoxides of the metals.
- It dissolved in concentrated hydrogen fluoride to form more soluble K2NbOF5 and less soluble K2TaF7.
- These are separated mostly by fractional crystallization.
- Reduction of K2NbOF5 by aluminum gives niobium.
Niobium Compounds
The +5 (V) oxidation state is most common for niobium. It shows a wide range of lower oxidation states which are not as unstable as zirconium and hafnium. However, the compounds of metal in lower oxidation states are unstable and reducing in nature.
It shows little cationic chemistry. The halides and oxohalides are the most important chemical compounds of the metal. These are mostly volatile and readily hydrolyzed.
Niobium Oxide
Niobium oxide, Nb2O5 is obtained during heating the metal or its stable compounds in the presence of oxygen. It is a white, high melting chemically inert solid practically insoluble in water or acids.
The various niobates are actually mixed oxides providing octahedral coordination around the metal.
Niobium Halide
The petahalides of niobium are obtained by direct reaction of the metals with halogens. For example, NbF5 may be obtained by the reactions of the metal or metal pentachloride with fluorine. These are white solids and soluble in organic compounds like ether or carbon tetrachloride.
They are hydrolyzed to form gelatinous hydrous oxide. The oxohalides of Nb are formed during the reaction of petahalides with oxygen.
Complex Compounds
The transition metal niobium formed different types of complex compounds in different oxidation states. It also formed peroxo and halo complexes. Some examples of metal complexes are given below the table,
| Complex compounds of Niobium | |||
| Oxidation state | Co-ordination number | Geometry | Examples |
| +5 (V) | 6 | octahedral | [NbCl5OPCl3] |
| trigonal prism | [Nb(S2C6H4)3]− | ||
| +4 (IV) | 6 | octahedral | [NbCl6]−2; [NbBr6]−2 |
| 8 | dodecahedral | [NbCl4(diars)2]; K4[Nb(CN)8]2H2O | |
| +3 (III) | 6 | octahedral | [Nb2Cl9]−3; [Ta2Cl6(SMe2)3] |
| 8 | dodecahedral | K5[Nb(CN)8] | |
Organometallic Compounds
Niobium forms a large number of organometallic compounds by M-C σ-bonds and η-C2H5 bonds. The methyl compound of the metal is also formed by the reduction of the pentahalides in the ether medium.
The alkyls are Lewis bases, adding neutral ligands or forming anions. It formed cyclopentadiene (CP) complexes like M(η5 – Cp)2(η1 – Cp)2.
What is Niobium Used For?
- Niobium is used in alloying metals for the production of stainless steel. The steel contains Nb used mostly in jet engines and rockets, beams, oil rigs, and gas pipelines.
- The alloy wires like Nb-Ge, Nb-Sn, and Nb-Ti are used in superconducting magnets. These types of alloys are also used in different instruments like particle accelerators, MRI scanners, and NMR spectrometers.
- In the production of surface acoustic wave devices for mobile phones and optical modulators, we also used the lithium niobate compound.
- The oxides of niobium are used to increase the refractive index of glasses. Therefore, these types of glasses are used for manufacturing thinner lenses.