Zirconium Metal
Zirconium is a chemical element or silvery-white, hard, and high melting transition metal of Group 4 (IVB) of the periodic table with the symbol Zr and atomic number 40. It is placed in d-block elements with group members titanium and hafnium. It is used for making bullet-proof steel and other corrosion-resistant alloys. The zirconium mineral zircon occurs generally in a beautiful range of colour like golden, orange, and pink. Therefore, these are used in gemstones from the early days of human civilization. The mineral zircon has two main forms, hyacinth and jargon. The early analysis showed it was the oxides of silicon, iron, aluminum, and calcium. The most common oxidation number or state of zirconium metal is +4 (IV). The lower oxidation states of the metal are less common.

Who Discovered Zirconium?
In 1789, the German chemist Klaproth discovered a new element from zircon and named it zirconium. However, the crude metal was first extracted in 1824 by Berzelius. Pure Zr metal was obtained by the van Arkel process in 1925.
Properties of Zirconium
The metal is fairly electropositive from dioxide on heating. The massive metal at room temperature is resistant to corrosion.
It dissolves only in hot concentrated sulfuric acid, aqua regia, and hydrofluoric acid. Hot aqueous alkali generally not attack this transition metal. Some common properties of zirconium are given below in the table,
| Properties of Zirconium |
|||
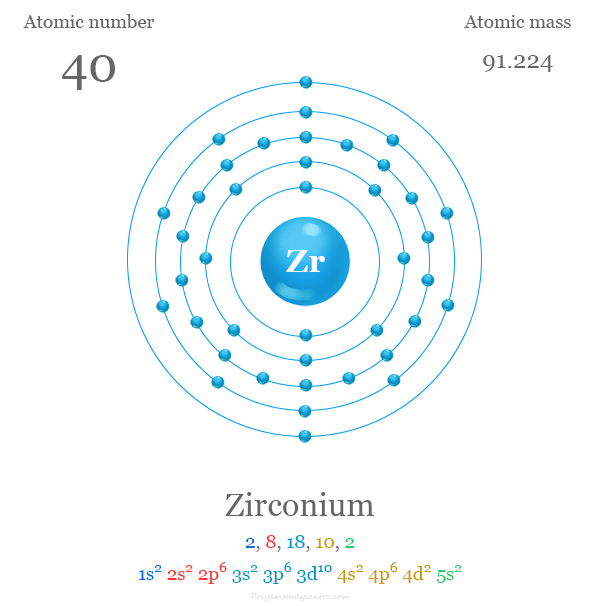
| Atomic number | 40 | ||
| Electron per shell | 2, 8, 18, 10, 2 | ||
| Electronic configuration | [Kr] 4d2 5s2 | ||
| Block | d-block | ||
| Period | period 5 | ||
| Group | group 4 | ||
| Atomic weight | 91.224 | ||
| Melting point | 2128 K (1855 °C, 3371 °F) | ||
| Boiling point | 4650 K (4377 °C, 7911 °F) | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 6.52 g/cm3 | ||
| Molar heat capacity | 25.36 J mol−1K−1 | ||
| Electrical resistivity | 421 nΩ·m | ||
| Atomic radius | 160 pm | ||
| Covalent radius | 175±7 pm | ||
| Chemical properties | |||
| Common oxidation number | +4 | ||
| Electronegativity | Pauling scale: 1.33 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 640.1 | 1270 | 2218 | |
Electron Configuration of Zirconium
The 40 electrons of the silver atom are distributed in different energy levels to show the following electronic configuration given below the picture,
Zirconium in the Periodic Table
It is placed in period 5 and group 4 in the periodic table. Zirconium is a transition metal or d-block element that lies between yttrium and niobium.
Where is Zirconium Found?
It is quite an abundant metal ranking after transition metals iron, titanium, and manganese. It contains 0.016 percent or 162 ppm of the earth’s crust.
The principal commercial source of zirconium is zircon (ZrSiO4) and Baddeleyite (ZrO2). The minerals are found generally in Australia, Brazil, India, Russia, South Africa, and the United States.
Isotopes
It has five isotopes 90Zr, 91Zr, 92Zr 94Zr, and 96Zr. The longest-live radioactive isotope of zirconium is 96Zr (2.4 × 1019 years).
Twenty-eight artificial isotopes of the metal ranging from atomic mass 78 to 110 can be prepared by different types of nuclear reactions.
Production Process
It is produced by the Kroll process. The minerals are converted to chloride during heating with carbon and chlorine. The tetrachloride is reduced with molten magnesium.
Very pure zirconium is produced generally by the van Arkel process. The metal is heated with iodine in an evacuated vessel at 200°C. The volatile ZrI4 decomposes on an electrically heated filament at 1300 °C. The hafnium content has been reduced below 100 ppm which can be achieved by solvent extraction or ion exchange methods.
Interesting Facts About Zirconium
The most common and stable oxidation state of zirconium is +4 (IV). However, Zr+3 is strongly reducing in nature which reduces water molecule. Hence in the +3 state, it has no aqueous chemistry.
Chemical Compounds
Zirconium Oxide
Zirconium oxide (ZrO2) is the most common solid oxide with a high melting point. It is insoluble in water, cold dilute acids (except HF), and alkalis. It reacts with acids slowly when heated for a long time and with alkali on fusion.
ZrO2 is used in making refractory crucibles and furnace linings. The white gelatinous hydroxide precipitate is obtained when adding an alkali to the Zr(IV) solution.
Zirconium Halides
All four tetrahalides of Zr are known and obtained when heating metal with halogens. All the halides are white solids. The unit cell of solid ZrF4 involves 2:8 coordination.
A ZrF4 unit is formed when a Zr atom is surrounded by eight fluorine ions in a square antiprism configuration. The tetrahalides are vigorously hydrolyzed at room temperature and produce oxohalides which are stable to further hydrolysis.
Zirconium Complexes
Zirconium (IV) nitrate and sulfate are the basic salts of the metal. The sulfate compound is formed by dissolving ZrO2 in hot concentrated sulfuric acid while the anhydrous nitrate may be prepared by reacting N2O5 on ZrCl4.
The large Zr+4 ion shows high coordination numbers and forms a variety of coordination complexes. Some coordination complexes of zirconium are given below in the table,
| Zirconium complexes | |||
| Oxidation state | Co-ordination number | Geometry | Examples |
| +4 (IV) | 6 | octahedral | Li2[ZrF6]; [Zr(acac)2Cl2] |
| 7 | pentagonal bipyramid | [ZrF7]-3 | |
| 8 | square antiprism | [ZrF8]-4; [Zr(acac)4] | |
| dodecahedral | [Zr(ox)4]-4; [ZrX4(diars)2]; [Zr4(OH)8(H2O)16]+8 | ||
Organometallic compounds
A few unstable hydrocarbon derivatives like Li[Zr(Me)6] have been isolated during the reaction of LiMe and ZrCl4 at low temperatures.
Zr(benzyl)4 is a stable and homogeneous chemical catalyst in the Ziegler-Natta process. Cyclopentadine (Cp) forms a number of important compounds comparable to that of titanium. These are used generally as homogeneous catalysts for the production of many organic compounds.
Uses of Zirconium
- Zirconium has a very low absorption of thermal neutrons but it may be free from traces of hafnium which absorbed neutrons 600 times more. Therefore, it is the ideal material used in nuclear power stations. The annual production of nuclear grade hafnium-free zirconium is all time high of about 165 tonnes.
- It is used to manufacture seamless coolant tubes for eight heavy water reactors.
- Zirconium is used in making bullet-proof alloy steels and other corrosion-resistant alloys.
- In many chemical plants, stainless steel is preferred over titanium. Zr-Sn alloys are also used in the cooler of nuclear reactors.
- Due to the corrosion resistance properties of acids and alkalis, it is widely used in the chemical industry.
- Like titanium, it is used in pumps, valves, heat exchangers, pipings, etc. It is used for making superconducting magnets when used with niobium
- It is used as a scavenger in the steel industry which removes dissolved oxygen and nitrogen.
- When the mineral zircon is mixed with vanadium and praseodymium, blue and yellow pigments are formed.
- Zirconium(IV) oxide is also used in making refractory crucibles, furnace linings, foundry bricks, abrasives, glass, and ceramics industries.
Analysis of Zirconium Metal
Zirconium precipitated in routine quantitative analysis of Group-3 or Group-IIIA metals. When added hydrogen peroxide (H2O2), a white precipitate of Zr peroxide (ZrO2, xH2O) appeared. Alizarin-S also gives a red precipitate with the solution of metal in a strongly acid medium. The colour is discharged by the action of fluoride.
Zirconium also formed a very characteristic phosphate Zr(HPO4)2 even from the solution which contains 10 percent sulfuric acid.