Tantalum Element
Tantalum is a chemical element or rare, hard, blue-gray, lustrous transition metal of Group 5 (VB) of the periodic table with the symbol Ta and atomic number 73. The element is relatively unreactive and resistant to all acids except hydrofluoric acid at ordinary temperatures. The transition element Ta also shows little cationic chemistry and forms a large number of anionic species. It is used for manufacturing capacitors and filaments in lamps due to its high density, extremely high melting point, and excellent corrosion resistance properties. Today, tantalum capacitors are used in different types of electronic devices like mobile phones, DVD players, video game systems, and computers. The high melting and boiling point of tantalum suggests the participation of a large number of d-electrons in metallic bonding.
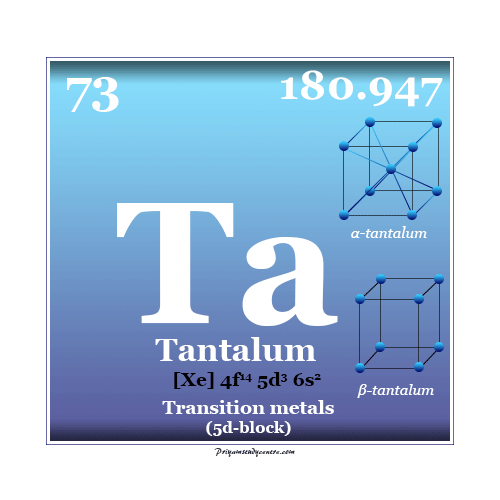
Properties of Tantalum
Pure tantalum forms a body-centered cubic crystal lattice. It has similar chemical properties to that of niobium.
| Tantalum |
||
| Symbol | Ta | |
| Discovery | Discovered by Anders Gustav Ekeberg in 1802 | |
| Name derived from | The legendary Greek figure King Tantalus | |
| Common isotopes | 73Ta180, 73Ta181 | |
| CAS number | 7440-25-7 | |
| Periodic properties | ||
| Atomic number | 73 | |
| Atomic weight | 180.947 | |
| Electron per shell | 2, 8, 18, 32, 11, 2 | |
| Electronic configuration | [Xe] 4f14 5d3 6s2 | |
| Period | 6 | |
| Group | 5 | |
| Block | d-block | |
| Physical properties | ||
| State at 20 °C | Solid | |
| Melting point | 3290 K (3017 °C, 5463 °F) | |
| Boiling point | 5731 K (5458 °C, 9856 °F) | |
| Density | 16.69 g/cm3 | |
| Crystal structure | α-Ta | β-Ta |
| Body-centered cubic crystal | Tetragonal crystal | |
| Molar heat capacity | 25.36 J mol−1 K−1 | |
| Electrical resistivity | 131 nΩ m | |
| Chemical properties | ||
| Atomic radius (non-bonded) | 2.22 Å | |
| Covalent radius | 1.58 Å | |
| Oxidation number or state | +5 | |
| Electronegativity | 1.50 (Pauling scale) | |
| Electron affinity | 31.068 kJ mol−1 | |
| Ionization energy (kJ/mol) | 1st | 2nd |
| 761 | 1500 | |
Electron Configuration of Tantalum
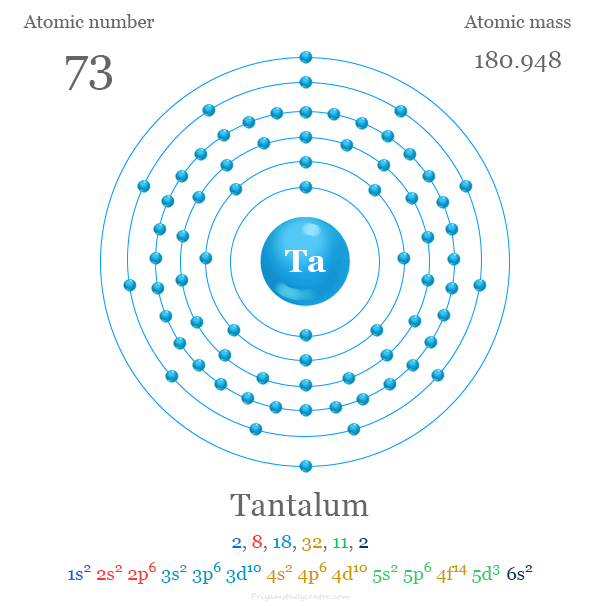
The element has [Xe] 4f14 5d3 6s2 valence shell electronic configuration. Therefore, tantalum is a d-block element or transition metal.
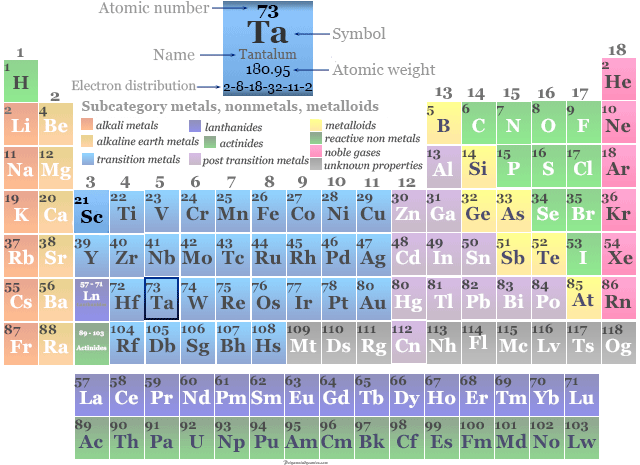
Tantalum on the Periodic Table
The element is placed in period 6 and group 5 of the periodic table. It lies with transition metals vanadium and niobium in the periodic table.
Who Discovered Tantalum?
In 1802, Anders Ekeberg discovered an oxide of a new metallic element in Sweden that could not be dissolved in any acids. He named the metal tantalum after Tantalus, a villain from Greek mythology.
One year earlier, Charles Hatchett had discovered columbium (now called niobium) from a mineral in North America.
In 1846, the German chemist, Heinrich Rose established that the chemical elements columbium and tantalum were chemically very similar and always occurred together. He renamed Columbium, niobium (Niobe was the daughter of Tantalus), which was adopted by IUPAC in 1950.
In 1864, Christian Wilhelm Blomstrand and Henri Etienne Sainte-Claire Deville observed the solubilities of potassium flrorotantalate and fluroniobate in hydrogen fluoride. The pure form of Nb and Ta was obtained in 1907 (Werner von Bolton) by the reduction of flurometallates by sodium.
Where is Tantalum Found?
Tantalum is found in the earth’s crust to the extent of 1.7 ppm. Owing to the great chemical similarities with niobium, tantalum always occurs with niobium.
The main mineral of the metal is tantalate with the chemical composition (Fe, Mn) M2O6, M = Nb, Ta. It is obtained mostly as a byproduct of the extraction of tin in Malaysia and Nigeria.
Isotopes
Tantalum has two natural isotopes 180mTa (m = metastable) and 181Ta with the occurrence of 0.012 percent and 99.988 percent respectively. These isotopes are obtained generally by the isomeric transition, beta decay, or electron capture nuclear reaction.
The radioactive half-life of these radioactive isotopes can not be observed experimentally. These isotopes are used for the production of different types of short-lived isotopes like 8Li, 80Rb, and 180Y.
Production Process
- The mineral tantalum is fused with potassium carbonate and extracted with water. The insoluble hydroxide of iron and manganese is largely eliminated by the process.
- The aqueous extract containing K3NbO4 and K3NbO4 is treated with carbon dioxide to precipitate the pentaoxides of the metals.
- It dissolved in concentrated hydrogen fluoride from more solube K2NbOF5 and less soluble K2TaF7.
- These are separated by fractional crystallization.
- Reduction of K2NbOF5 by aluminum gives niobium. Electrolysis of molten K2TaF7 gives tantalum.
Nowadays, metals are also produced by the solvent extraction process.
Facts About Tantalum
- The effect of lanthanide construction makes the metallic and ionic radii of niobium and tantalum the same.
- The chemistry of these two metals is very similar but the ground electronic configurations are different.
- The stable and common oxidation state of Ta is +5 (V) but it shows a wide range of lower oxidation states.
- The lower oxidation compounds of Ta are relatively more stable than zirconium and hafnium.
Chemical Compounds
The halides and oxohalides are the most important chemical compounds of tantalum. They are mostly of a volatile nature. A large number of metal atom cluster compounds are also known in the lower oxidation state of the metal.
Complexes of Tantalum
The main chemical complexes in the different oxidation states of tantalum are proxy and halo complexes. The oxidation state, geometry, coordination number, and examples of such complexes are given below in the table,
| Tantalum compounds | |||
| Oxidation state | Co-ordination number | Geometry | Examples |
| +5 (V) | 6 | octahedral | [TaF6]−; [TaCl5, SMe2] |
| trigonal prism | [Ta(S2C6H4)3]− | ||
| +4 (IV) | 6 | octahedral | [TaCl6]−2; [TaBr6]−2 |
| 8 | dodecahedral | [TaCl4(diars)2] | |
| +3 (III) | 6 | octahedral | [Ta2Cl6(SMe2)3] |
Organometallic Complexes of Tantalum
It has extensive chemistry with organic compounds when involving Ta-C σ-bonds and η-C2H5 bonds. The methyl forms of the metal are stable and generally prepared by the reduction of the penta-halides in an ether medium.
TaMe5 may explode spontaneously at room temperature. The alkyls are Lewis bases that react with natural ligands or form anions.
Uses of Tantalum
- Tantalum is used mostly for the production of electronic components, mainly in capacitors, high-power resistors, and filaments in Lamps.
- An insulating or dielectric oxide layer forms on the surface of Ta. Therefore, the metal used to coat other metals and a high capacitance can be achieved. Due to their small size and weight, tantalum capacitors are used in mobile or portable telephones, laptops, automotive electronics, and cameras.
- Tantalum is highly inert to the body fluids and used or suitable for making skull plates, screws, and wires used in orthopedic surgery.
- It is also used for the production of different types of alloys that have high melting points, strength, and ductility.
- Tantalum alloys are used mostly in turbine blades, rocket nozzles, and nose caps for supersonic aircraft.
- It is highly inert toward most acids and alkalies except hydrofluoric acid and hot sulfuric acid. Therefore, it is used for making chemical reaction vessels and pipes for corrosive liquids.
- Tantalum has been also used as an electrode in neon lights, and AC/DC rectifiers.