Chlorine Dioxide (ClO2)
Chlorine dioxide (chemical formula ClO2) is a yellowish-green gas or disinfectant always handled in an aqueous solution and uses mainly for the treatment or production of drinking water. It is a toxic chemical compound that has a pungent odor similar to chlorine and nitric acid. The chemical compound chlorine dioxide has serious side effects when uses in large amounts. Commercially, chlorine dioxide is generated or made by reacting sodium chlorate with sulfur dioxide or hydrochloric acid in a strong acid medium. Chlorine dioxide is soluble in water to form a yellowish-brown solution. Therefore, chlorine dioxide does not hydrolyze when it enters water but strong acids can decompose it into chlorine and oxygen.
The yellow gas condensed to a reddish-brown liquid between 11 °C. We used very small quantities of chlorine dioxide for the disinfection of drinking water. When used in large quantities, it should be dangerous for human health.
Chlorine Dioxide Generation
The explosive gas chlorine dioxide may be prepared or generated by heating potassium chlorate at 90 °C with moist oxalic acid. The liberated carbon dioxide can dilute the explosive gas. Therefore, it makes advantages in the generation of chlorine dioxide.
2KClO3 + 2H2C2O4 → 2ClO2 + 2CO2 + K2C2O4 + 2H2O
Chlorine Dioxide From Sodium Chlorite
Generally, chlorine dioxide can be prepared in the laboratory by oxidation of sodium chlorite (NaClO2) solution with chlorine, hydrochloric acid, or sulfuric acid. Very pure ClO2 can be generated when solid sodium chlorite reacts with dilute chlorine gas.
NaClO2 + ½Cl2 → ClO2 + NaCl
5NaClO2 + 4HCl → 4ClO2 + 5NaCl + 2H2O
4NaClO2 + 2H2SO4 → 2ClO2 + HClO3 + 2Na2SO4 + H2O + HCl
Among these three oxidizing agents, sulfuric acid can generate completely chlorine-free ClO2.
Generation from Sodium Chlorate
Commercially, the chlorine dioxide gas is generated on a massive scale by reducing sodium chlorate (NaClO3) solution with a suitable reducing agent such as methanol, hydrogen peroxide, or sulfur dioxide in a strong acid medium. Hydrochloric acid may also used as a reducing agent but hydrochloric acid contaminates the product with chlorine.
2 NaClO3 + SO2 + H2SO4 → 2 ClO2 + 2 NaHSO4
2 ClO3− + 2 Cl− + 4 H+ → 2 ClO2 + Cl2 + 2 H2O
Methanol or hydrogen peroxide is the best reducing agent in the ClO2 generation process because they do not produce any elemental chlorine gas.
Pure Chlorine Dioxide
Pure ClO2 is obtained by the reduction of silver chlorate with dry chlorine gas. The evolved ClO2 gas condensed to a liquid.
2 AgClO3 + Cl2 → 2 AgCl + 2 ClO2 + O2
For the generation of very pure chlorine dioxide, electrolysis of a chlorite solution is carried out in an electrolytic cell. It generates ClO2 at the anode and hydrogen at the cathode.
NaClO2 + H2O → ClO2 + NaOH + 1⁄2H2
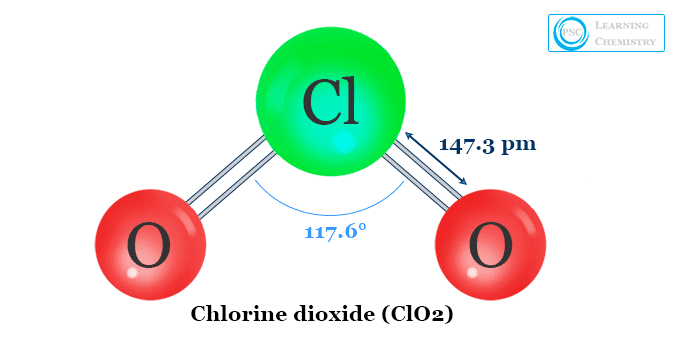
Structure of Chlorine Dioxide
Chlorine dioxide (ClO2) formed a bent type structure with an O-Cl-O bond angle of 117.6°. The O-Cl bond distance in the ClO2 molecule is 149 pm. It is slightly shorter than a single bond. Pauling represents the molecule that contains a resonance hybrid with three electron structures.
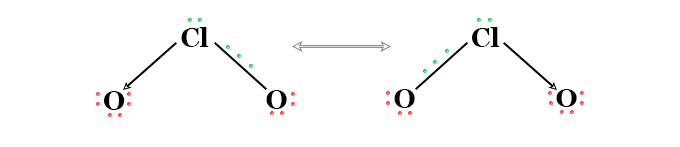
An alternative scheme of the three-electron structures with the odd electron residing on an oxygen orbital may also be worked out. The resonating hybrid is compatible with the sp3d hybridization of the chlorine atom.
- One orbital has lone pairs and another makes the coordinate link to one oxygen atom.
- The third and fourth sp3d orbital makes a sigma and a pi bond with other oxygen atoms.
- The odd electrons remain located on the fifth sp3d orbitals of the ClO2 molecule.
It has (2 × 6 + 7) = 19 odd electrons and a tendency to dimerize. No evidence of dimerization in the solid, liquid, or gas phase or solution.
Properties
Physical Properties
At standard temperature and pressure, chlorine dioxide is a reddish-yellow gas that is soluble in water to form a yellowish-brown solution. The gas is 10 times more soluble in water than chlorine gas.
Chlorine dioxide is an unstable irritating gas or solution that decomposes chlorine and oxygen in the presence of strong acids. It is a toxic gas that has various types of side effects on human health. Hence we need to maintain safety before exposure to ClO2 in laboratories or production plants. Many times, we generate it at the working site because the gas or solution of ClO2 is highly explosive for transportation.
The most common properties of chlorine dioxide are given below in the table,
| Properties | |
| IUPAC name | Chlorine dioxide |
| Chemical formula | ClO2 |
| Molar mass | 67.45 g/mol |
| Appearance | Yellow to reddish toxic gas and yellowish-brown solution in water |
| Density | 2.757 g/dm |
| Melting point | −59 °C |
| Boiling point | 11 °C |
| Solubility | Soluble in water, also soluble in alkaline and sulfuric acid solutions |
| Solubility in water | Highly soluble in water to form a solution (8 g/L at 20 °C) |
| CAS Number | 10049-04-4 |
Chemical Properties
ClO2 is soluble in water to form dark green or yellowish brown solutions up to 8 g/L in dark conditions. In the presence of light, ClO2 decomposes solely to form HCl and HClO3. Crystalline clathrate hydrate may be obtained by cooling the solution.
When dissolved in sodium hydroxide or potassium hydroxide, it forms chlorite and chlorate.
2 ClO2 + 2 NaOH → NaClO2 + NaClO3 + H2O
2 ClO2 + 2 KOH → KClO2 + KClO3 + H2O
It is a powerful oxidizing and chlorinating agent. Aqueous suspensions of powdered metals (Mg, Zn, Ni, Cd, and Al) are converted to their chlorites by ClO2 at ordinary temperature and pressure.
Mg + 2 ClO2 → Mg(ClO2)2
Al + 3 ClO2 → Mg(ClO2)3
Ozone can be oxidized ClO2 to Cl2O6.
2 ClO2 + O3 → Cl2O6 + 2 O2
Alkaline hydrogen peroxide should be reacted with ClO2 to give sodium chlorite (NaClO2). The chemical sodium chlorite is used for belching textiles and paper pulp.
2 ClO2 + 2 NaOH + H2O2 → 2 NaClO2 + O2 + 2 H2O
Uses of Chlorine Dioxide
Chlorine dioxide is a powerful oxidant and disinfectant used commonly for the disinfection of municipal drinking water. Huge quantities of ClO2 are produced for bleaching wood pulp, cellulose, and textiles products. It is also used for generating sodium chlorite (NaClO2). It can be used in manufacturing sites due to its explosive nature.
For Cleaning
Chlorine dioxide has several cleaning applications.
- In the electronic industry, ClO2 solution is used to clean circuit boards.
- Chlorine dioxide gas and solution are uses for sterilizing and cleaning medical and laboratory equipment, surfaces, rooms, and tools.
- Mouthwash containing small quantities of chlorine dioxide can improve bad breath. When we take it in the mouth, it is unsafe for our health. But it is possibly safe when we use very small quantities of ClO2 in mouthwash.
Bleaching
Present-day, chlorine dioxide has been uses widely in industry for bleaching paper and textile products. It is an effective and stronger cleaner for fiber than chlorine. During bleaching, it does not produce harmful byproducts such as organochlorine compounds.
Disinfection with Chlorine Dioxide
Chlorine dioxide should be used widely as a disinfectant or oxidant. It is a very strong oxidizer and effectively works in many disinfection processes. It kills pathogenic microorganisms such as fungi, bacteria, and viruses.
We use ClO2 instead of chlorine because it prevents the formation of harmful halogenated disinfection byproducts such as trihalomethanes and halogenated acidic acids. Therefore, ClO2 generating systems are used in the following disinfection process:
- Drinking water treatment or disinfection is the main application of chlorine dioxide. Therefore, during disinfection of water, it fights against the impurities of water.
- It effectively sanitizes molds and yeast from several fruits such as blueberries, raspberries, and strawberries
- It can disinfect and fight against parasites like Giardia Lambia and Cryptosporidium.
- The spray of ClO2 may be used to disinfect or sterilize poultry farms.
- For swimming pool disinfection, we used a combination of chlorine and ClO2.
- It is used to disinfect the water that flows through cooling towers.
Chlorine Dioxide for Water Treatment
A small amount of ClO2 solution is less corrosive than chlorine and significantly controls several types of bacteria in the water treatment process. It is a very good water disinfectant than other secondary disinfectants. Therefore, ClO2 uses in many water treatment plants such as cooling towers, water, and food processing plants.
In 1956, chlorine dioxide was introduced on a large scale for disinfection of drinking water. It was primarily used for removing inorganic components such as manganese and iron. It has no taste or odor in water and destroys taste or odor-producing phenolic compounds. In drinking water treatment, ClO2 acts as a disinfectant and oxidizing agent.
- It can be used for treating water in pre-oxidation and post-oxidation steps. In all the stages, ClO2 should be used for chlorination in drinking water to destroy natural water impurities or prevent the growth of algae and bacteria.
- It is a powerful disinfectant for several types of bacteria and viruses. It helps to prevent the growth of bacteria in municipal water supply systems.
- It should be active against biofilm in the water distribution system. Biofilm is a protective layer of pathogenic microorganisms which does not defeat by most common water disinfectants. It has the ability to destroy or kill the pathogenic microorganisms layer.
Side Effects
The supplements which contain chlorine dioxide are likely to be unsafe for human health. We used a very small amount of ClO2 for the disinfection of drinking water used in the public sector. Acute ClO2, exposure can form chlorine gas which causes irritations and burns the skin and eyes. High absorption of ClO2 can damage the tissues and blood cells of our skin.
Chlorine dioxide has many serious side effects when used in large amounts or inhalation. Inhalation can cause serious toxic side effects such as coughing, sore throat, severe headaches, vomiting and diarrhea, liver failure, and death. Therefore, always handle safely when you work with this compound.
Frequently Asked Questions
What is Chlorine Dioxide?
Chlorine dioxide (chemical formula Cl2O) is a yellowish-green gas or reddish-brown solution that is uses in very small quantities to disinfect water. The disinfectant ClO2 is similar to a powerful bleaching agent but unsafe when used in large amounts.
Commercially, ClO2 is generated by reacting sodium chlorate with sulfur dioxide or hydrochloric acid in a strong sulfuric acid medium. It does not hydrolyze when it enters water but strong acids can decompose it into chlorine and oxygen.
What is Chlorine Dioxide Used For?
Chlorine dioxide is a powerful oxidant and bleaching agent or disinfectant uses in very small quantities for the deodorizing and purifying of drinking water. It is commonly added to drinking water to protect ourselves from harmful bacteria and other microorganisms. Therefore, ClO2 generating systems can be used for odor control, sanitation, and water purification processes.
Huge quantities of ClO2 have been generated and uses for bleaching wood pulp, cellulose, food products, textiles products, and microbiological control in cooling towers. It is also used for the generation of sodium chlorite (NaClO2). Many times, it can be generated in working sites because the gas form or solution of ClO2 is highly explosive for transportation.
Is Chlorine dioxide Bleach?
Yes, chlorine dioxide solution has uses for bleaching wood pulp, cellulose, food products, textiles products, and microbiological control in cooling towers. It has powerful oxidizing properties and uses in very small quantities for the deodorizing and purifying of drinking water.
How to Make Chlorine Dioxide?
Commercially, the chlorine dioxide gas or solution is made on a massive scale by using sodium chlorate with a suitable reducing agent such as methanol, hydrogen peroxide, or sulfur dioxide in a strong acid medium.