Manganese Chemical Element
Manganese is a chemical element or a greyish-white hard, brittle paramagnetic transition metal of Group 7 (VIIB) of the periodic table with the symbol Mn and atomic number 25. It is used mostly as an additive to steel. The Swedish chemist Bergmann discovered the presence of manganese in the black magnesia but failed to isolate it. In chemistry, the element was isolated by another Swedish chemist Johan Gottlieb Gahn, and studied by the Swedish chemist Carl Wilhelm Scheele in 1774. The metal was named in the Latin word magnesium from the old name pyrolusite. It formed a body-centered cubic crystal lattice. The melting point and density of manganese are much lower than those of chromium suggesting more participation of d-electron in metallic chemical bonding.
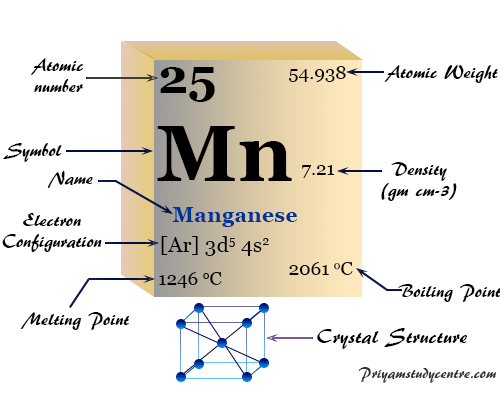
Properties of Manganese
The +6 oxidation number or state is familiar to us in the compound potassium permanganate, KMnO4, a strong oxidizing agent.
| Manganese | |||
| Symbol | Mn | ||
| Discovery | Johan Gottlieb Gahn in 1774 | ||
| Name derived from | The Latin magnes means magnet or from the black magnesium oxide magnesia nigra | ||
| Common isotope | 55Mn | ||
| Oxidation states | −3, −2, −1, 0, +1, +2, +3, +4, +5, +6, +7 | ||
| CAS number | 7439-96-5 | ||
| Periodic properties | |||
| Atomic number | 25 | ||
| Relative atomic mass | 54.938 | ||
| Electron per cell | 2, 8, 13, 2 | ||
| Electronic Configuration | [Ar] 3d5 4s2 | ||
| Block | d-block | ||
| Group | 7 | ||
| Period | 4 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1246 °C, 2275 °F, 1519 K | ||
| Boiling point | 2061 °C, 3742 °F, 2334 K | ||
| Molar heat capacity | 26.32 J mol−1 K−1 | ||
| Crystal structure | body-centered cubic (bcc) | ||
| Density | 7.3 g/cm3 | ||
| The heat of fusion | 12.91 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.05 Å | ||
| Covalent radius | 1.29 Å | ||
| Electronegativity | 1.55 (Pauling scale) | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 717.27 | 1509.03 | 3248.47 | |
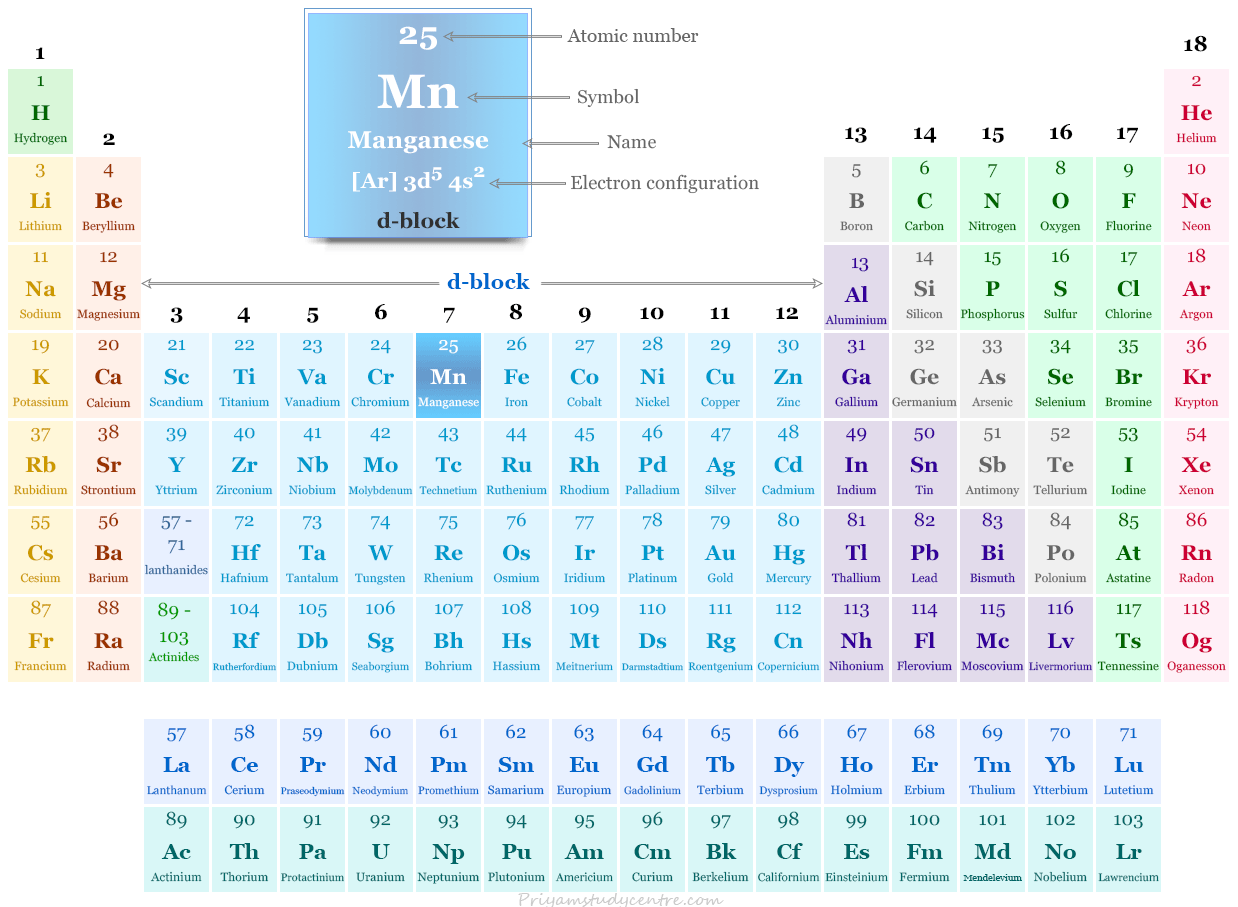
Manganese on the Periodic Table
It is placed in group 7 and period 4 with d-block elements in the periodic table. Manganese is a member of a transition metal that possesses the outer orbital electronic configuration [Ar] 3d5 4s2.
Where is Manganese Found?
It is the third most abundant transition metal after iron and titanium and the twelfth most abundant element among all periodic table elements. It occurs at 1000 ppm in the earth’s crust.
Manganese is found in a variety of minerals like pyrolusite (MnO2) Hausmannite (Mn3O4), Braunite (Mn2O3) Manganese spar (MnCO3) in Russia, India, South Africa, Ghana, Brazil, and Chile.
Colloidal particles of manganese are found on the ocean floor to form compact Mn nodules. It generally contains 15 to 30 percent of metal on a dry basis with copper, nickel, and cobalt. More than 1012 tones of such nodules have already been accommodated from the ocean water bed. The large deposit of the elements may gain commercial significance in the future.
The transition element, manganese present in food plants and animal bodies and plays an important role in the biological process.
Isotopes of Element
Naturally, occurring manganese has one stable isotope with an atomic mass of 55. It has also several radioactive isotopes with atomic mass ranges from 44 to 69.
The radioactive isotopes of Mn are obtained from different types of nuclear reactions. 55Mn (half-life = 3.7 million years) and 54Mn (half-life = 312.2 days) are the most stable isotopes of Mn.
Production Process
The chemical element, manganese is produced by electrolysis of an aqueous solution of Mn (II) sulfate which is prepared from pyrolusite by heating concentrated sulfuric acid at 150 °C.
Alternatively, pyrolusite is heated below 800 °C with dehydrated green vitriol. In both techniques, manganese sulfate (MnSO4) is leached out from the residue by leaving impurities like iron oxide and other silicate compounds.
The Mn (II) sulfate was further purified by crystallization before subjecting electrolysis.
Chemical Compounds
The greyish-white paramagnetic metal, manganese does not oxidize in a very pure form but the presence of impurities like carbon makes it more reactive.
It liberated hydrogen from all dilute acids, including dilute nitric acid. The acids are reduced to form sulfur dioxide and nitrogen monoxide (NO).
The +2 oxidation state is most stable due to the presence of a half-filled d5 electron configuration. The reduction potential oxidation state diagram suggests that Mn (VI) is a highly oxidizing agent, that oxidizes to form Mn (III) and Mn (IV) ions.
Potassium Permanganate
Potassium permanganate is an important compound of manganese having the chemical formula KMnO4. Permanganate is familiar to us due to its strong oxidizing properties.
Permanganate ion is a very strong oxidizing agent in acid solution and moderately strong in the neutral and alkaline medium. Therefore, potassium permanganate is used as an oxidizer in analytical chemistry.
In the +7 state, the metal forms oxides like Mn2O7 and oxohalides MnO3Cl and MnO3F. The green oxide is highly explosive and ignites alcohol or ether when brought into contact.
Manganate Ion
Manganate ion (MnO4−2) is the only suitable representative in the +6 state of manganese.
It is obtained as a green mass when fusing pyrolusite with alkali. The green mass is extracted with water containing little alkali and the solution is evaporated to yield a dark green crystalline solid K2MnO4.
Manganese (IV) Oxide
In the +4 state, manganese forms stable dioxide MnO2, halide MnF4, and the complexes MnX6−2, where X = fluorine, chlorine, cyanide, and IO3.
Pyrolusite (MnO2) is a common mineral present in the earth’s crust. It is the main source of the metal manganese. It is a slightly brownish-black solid mostly insoluble in water and inert to cold acids.
Hydrochloric acid and heated concentrated sulfuric or nitric acid are oxidized by MnO2. Manganese dioxide is an important compound of Mn, widely used glass industry to decolorize glass. It is also used in the manufacturing of match boxes.
Manganese (II) Compounds
The +2 state is the most common and stable oxidation state of manganese to form a large number of binary compounds (oxides, halides) and complexes.
The oxide, MnO prepared by getting MnO2 with hydrogen or posting MnCO3 with hydrogen or nitrogen. It is readily oxidized in air at ordinary temperature to form Mn2O3 or Mn2O4.
All four halides of manganese with the chemical formula MnX2 are stable compounds. They are also isomorphous with the magnesium halides. The halides are conveniently prepared during the reaction of MnCO3 with the appropriate hydracids of fluorine, chlorine, bromine, and iodine.
Transition Metal Complexes
In chemistry, manganese (II) forms an extensive range of complex compounds with different types of stereochemistry. The d5 configuration of manganese offers no crystal field stabilization energy (CFSE) in high-spin octahedral and tetrahedral complexes.
In the +2 oxidation state, it also forms stable high-spin octahedral complexes by ammonia, EDTA, oxalate, ethylenediamine, and SCN−.
The chemical equilibrium constants for the formation of these ions from aqua-ion are very low, which may again be attributed to the absence of any gain in CFSE.
Uses of Manganese
Uses of Manganese Steel
The transition element, manganese is mostly used as a chemical additive for making steel. Manganese combines with the sulfur present in steel which prevents the formation of brittle materials like FeS.
- Steel alloys with 70 to 80 percent manganese are known as ferromanganese.
- 15 to 20 percent metal is called spiegeleisen.
Due to their high strength, the manganese alloys have great industrial demand. They are used for making railway tracks, safes, rifle barrels, crushing machines, drilling rods, and prison bars.
The other alloys that generally contain 17 to 19 percent chromium, 8 to 10 percent manganese, 0.75 to 1 percent copper, and small amounts of carbon and silicon have high corrosion resistance and chemical attack.
Common Uses
- Several non-steel alloys like manganese bronze alloy are used for making propeller blades and manganin in electrical instruments due to their electrical resistivity.
- Manganese element and its chemical compounds are widely used as a chemical catalyst.
- It is also used in the pharmaceutical supplement, fertilizer, and glass industry as a decolorizer.
- In electrochemical cells or dry cells, elemental manganese is used as a depolarizer.