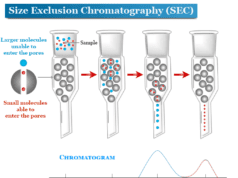
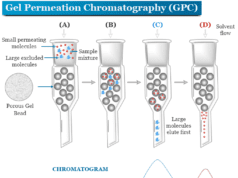
Column Chromatography Principle
Column chromatography is an analytical procedure or principle for the separation and purification of inorganic and organic compounds. We use column chromatography mostly for the purification and separation of organic molecules such as protein, pigment, amino acid, enzyme, and starch. Column chromatography can be used to separate substances based on differential adsorption of compounds to the adsorbent like activated alumina or silica gel. It is an extremely time-consuming technique in any lab but we used column due to its low cost. We also used the automatic column chromatography technique for the separation and purification of compounds. Solvent selection is the most important step in the column chromatography procedure because the affinity of adsorption depends on the selection of solvent.
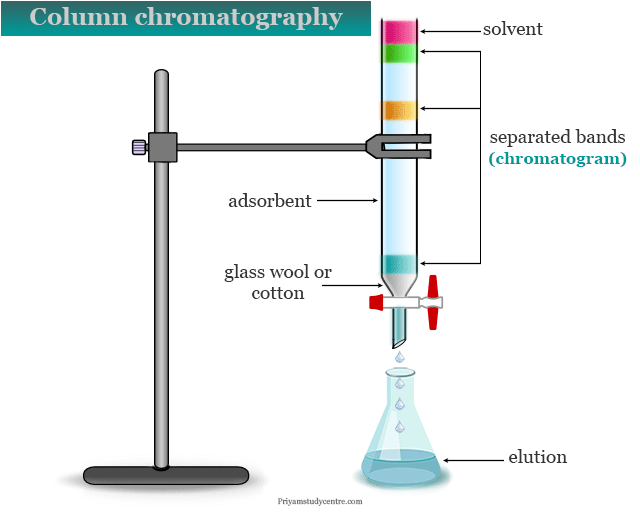
Column chromatography is the most widely used and user friendly separation technique that helps in the separation, identification, and purification of the components of a mixture for quantitative and qualitative analysis. It is based on the adsorption, partition, or ion exchange phenomenon.
Based on the flow rate, column chromatography is classified into the following five categories,
- Gravity chromatography
- Flash column chromatography
- Low or medium pressure chromatography
- Vacuum chromatography
- High-pressure/high-performance liquid chromatography (HPLC)
Column Chromatography Procedure
The size of the column is always chosen according to the sample that we separated. The smaller the diameter of the column, the more effective will be the separation. Therefore, a thin Pyrex walled glass tube of 15 to 20 cm in length and 1 cm in diameter is more effective for separation.
Column chromatography procedure is used for separation of a mixture of different compounds by following steps,
- Adsorbent
- Solvent selection
- Packing of column
- Sample placement
- Chromatogram development
Adsorbent
Activated alumina (Al2O3) and silica gel (SiO2) are the most commonly used and commercially available adsorbents in the column chromatography procedure. Activated alumina and charcoal are strong adsorbents. Calcium carbonate, calcium phosphate, magnesia, and shake lime are examples of intermediate adsorbent materials.
Organic compounds such as sucrose, starch, and insulin act as weak adsorbents. The absorbability of organic compounds depends on the nature and number of the polar groups present in the particular compounds. A suitable absorbent in such type of chromatography method has the following criteria,
- Absorbent particles have uniform shapes and sizes in the range of 60-200 μm in diameter.
- The absorbent has high mechanical stability and chemical inertness.
- The compound itself is colourless, inexpensive, and readily available.
- It has free passage for the mobile phase.
- It should be suitable for a wide range of samples.
Column Chromatography Solvent Selection
Adsorption depends on the selection of solvent in the column chromatography procedure. A relatively nonpolar solvent is used for placing the solute in a column but a polar solvent is used for developing a chromatogram.
The polarity of the solvent gradually increases by eluting absorbed material. The rates of elution are controlled by varying activities of absorbent and polarities of solvent. It is purely a trial and error procedure.
If the compounds are eluted rapidly, poor separation is obtained. We used milder absorbent and slightly polar solvent for the slow elution procedure. Therefore, we select nonpolar solvents for weakly absorbed compounds and polar solvents for strongly absorbed compounds in the column chromatography procedure.
The polarity and selection of solvent are the most important steps in the column chromatography procedure. The order of polarity of commonly used solvents is as follows,
Petroleum ether < carbon tetrachloride < cyclohexane < carbon disulfide < ether < acetone < benzene < ethyl acetate < chloroform < ethanol < methanol < water < pyridine < acetic acid.
Packing of Column Chromatography
Packing of the silica gel column in the chromatography procedure is most important due to avoiding air bubbles and errors in separation. The column tube is cleaned and dried perfectly.
When the solvent is run, it should not be allowed to dry until the experiment is complete. Columns in chromatography are prepared by dry packing or wet packing.
Dry Packing in Column Chromatography
We used a glass tube fitted with a stopper in dry packing. Some glass wool or cotton wool is placed at the top of the constricted portion of the glass tube by using a glass rod. The glass allows solvent to pass but retains the absorbent in the column which is placed in a vertical position.
A weighted amount of absorbent is added to a small portion of the tube for the setting of absorbent. The column is washed with eluting solvent before we use it. A disc of filter paper is placed to protect the surface of the glass tube.
Wet Packing in Column Chromatography
- In wet packing of column chromatography, a glass tube is clamped vertically by keeping the glass tube at the constricted end.
- The Haft of the tube is filled with eluting solvent.
- A mobile slurry of absorbent prepared from eluting solvent.
- The stopper of the column is opened to settle down the absorbent.
- The process is repeated until the whole slurry has been added.
- The top portion of the surface is protected by placing a disc of filter paper on it.
Sample Placement
The sample placed in the column is either a solvent solution or a direct liquid mixture of the sample. If a solid sample is insoluble in a nonpolar solvent, we used a polar solvent.
It is poured into a small amount of adsorbent and taken in a porcelain basin to prepare the thick slurry. The solvent is completely evaporated by stirring with a glass rod when the slurry turns power form.
Chromatogram
A solution of the sample mixture in a suitable solvent is allowed to pass down slowly to the column. When the individual components are selectively absorbed, a chromatogram is displayed.
It is a coloured or colourless band or zones in the column chromatography procedure. Therefore, the individual coloured band or zones are separated with organic solvents and collected in a 25 ml or 50 ml conical flask.
If the bands in the chromatogram are colorless, a quartz column is used instead of a glass column. It is placed in the light of a mercury lamp to detect the band with fluorescence. The colurless compounds may also be detected by the reagents which formed colour with the components of the sample mixture.
Separation by Column Chromatography
Column chromatography procedure is widely used for the separation,
- Naphthalene from phenol
- Anthracene from anthracene picrate
- Methyl orange from methyl blue
- Leaf pigments (chlorophyll from carotenoids)
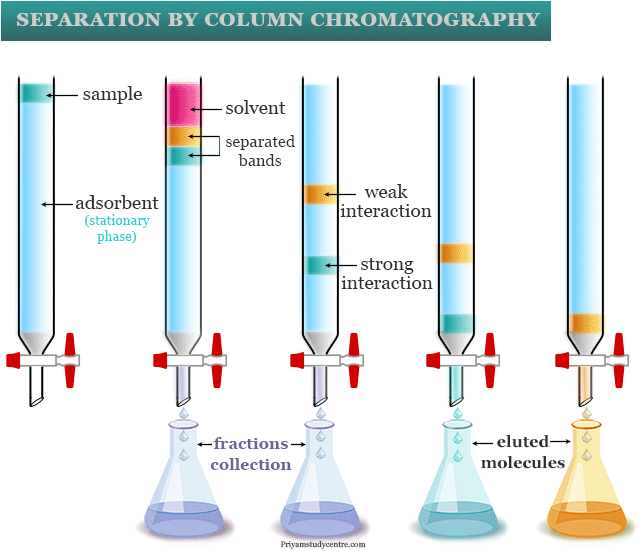
Separation of Organic Compounds
- A column of alumina is prepared by wet packing using hexane as a solvent for the separation of naphthalene from phenol by a chromatographic column. Phenol is absorbed strongly on alumina owing to intermolecular hydrogen bonding and is less soluble in nonpolar hexane.
- Other hand, naphthalene is absorbed weakly in the alumina due to more solubility in hexane. Therefore, these mixtures can easily be separated by chromatographic columns.
We also used the column chromatography principle for the separation of organic compounds such as anthracene from anthracene picrate and methyl orange from methyl blue.
Anthracene picrate is strongly absorbed by the alumina column but anthracene is weekly absorbed. Similarly, methyl orange will be absorbed strongly due to the presence of the -SO3H group but methylene blue will be weakly absorbed.
Separation of Leaf Pigments
A column of alumina is prepared by wet packing using ligroin solvent for the separation of leaf pigments by column chromatography procedure.
Leaf pigments contain mainly chlorophylls and carotenoids. Carotenoids are hydrocarbons containing isoprene groups or their oxy or hydroxy derivatives. Chlorophyll has two types, Chlorophyll-a and Chlorophyll-b.
- Carotenoids are nonpolar compounds that are weakly absorbed by alumina. Therefore, they are readily eluted with hydrocarbon solvents.
- Chlorophylls have several polar sites per molecule. Therefore, they are strongly absorbed by alumina and easily eluted with a polar solvent.
Column Chromatography for Protein Purification
Column chromatography is one of the most common procedures for the separation and purification of protein. Generally, proteins are long-chain flexible amino acids. They are either hydrophobic or hydrophilic, depending on the relative amount of amino acids which they contain.
For protein separation and purification, a column is packed with small beads that are coated with ion exchange resin. It attracts hydrophobic amino acids to the resin beads.
A series of solvents with different concentrations can be used to separate or purify many proteins from each other by column chromatography procedure.
Affinity Column Chromatography
The affinity column chromatography procedure is based on the exceptional ability of biological substances such as proteins to bind specifically and reversibly to other substances like affinity ligands. It is used mainly for the purification and separation of biological macromolecules.
Biological macromolecules can be easily released from affinity complexes by dissolving or changing the concentration of the solvent. Such dissociation may also be possible by altering pH scale, temperature, and ionic concentration.
Isolation, purification, and separation of protein, stretch, antibiotic, and enzyme are done by affinity column chromatography procedure or principle.