Lead Metal
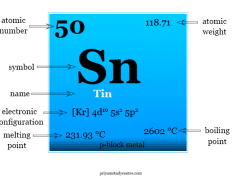
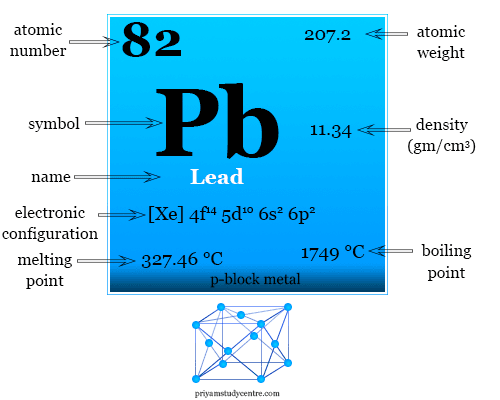
Lead is a chemical element or group-14 metal of the periodic table with the symbol Pb and atomic number 82. It has been used in plumbing in Roman civilization. Now, it has been stopped due to lead poisoning. Lead is used for making storage batteries and antiknock compound (lead tetraethyl) to control air pollution. It exists in a cubic closed-packed crystal lattice with a density of 11.34 g/cm3. Lead is a soft, malleable post-transition metal. The low melting point of lead suggests the fact that all four valence electrons in Pb do not participate in metallic bonding. +2 oxidation number is common for lead rather than +4.
Where is Lead Found?
It is found at 13 to 16 ppm in the earth’s crust. Galena (PbS) is the main source of lead metal. The other minerals which contain Pb(II) are anglesite (PbSO4) and cerussite (PbCO3).
The United States, Russia, Australia, and Canada are the major producers of Pb metal. In India, galena contains about 3 percent of Pb and a small amount of Ag. It is obtained from the Zawar mines at Udaipur in Rajasthan.
Properties of Lead
The chemistry and properties of group-14 elements follow from their electronic configuration. All the elements have an outermost quantum shell with an s2p2 configuration.
Lead exists in a face-centered cubic close-packed crystal lattice with a density of 11.34 g/cm3. It is a low-melting metal because it cannot use all four valence electrons for metallic bonding.
| Lead |
|||
| Symbol | Pb | ||
| Discovery | Ancient | ||
| Name derived from | Anglo-Saxon word for the metal, lead | ||
| common isotope | 82Pb208 | ||
| Periodic properties | |||
| Atomic number | 82 | ||
| Atomic weight | 207.2 | ||
| Electron per shell | 2, 8, 18, 32, 18, 4 | ||
| Electronic configuration | [Xe] 4f14 5d10 6s2 6p2 | ||
| Group | 14 | ||
| Period | 6 | ||
| Block | p-block | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 327.46 °C | ||
| Boiling point | 1749 °C | ||
| Density | 11.34 g/cm3 | ||
| Molar heat capacity | 26.650 J mol−1 K−1 | ||
| Electrical resistivity | 208 nΩ m | ||
| Crystal structure | Face-centered cubic crystal | ||
| Chemical properties | |||
| Atomic radius (non-bonded) | 2.02 Å | ||
| Covalent radius | 1.45 Å | ||
| Common oxidation number | +2, +4 | ||
| Electronegativity | 1.8 (Pauling scale) | ||
| Electron affinity | 35.12 kJ mol−1 | ||
| Ionization energy (kJ/mol) |
1st | 2nd | 3rd |
| 715.6 | 1450.5 | 3081.5 | |
| CAS number | 7439-92-1 | ||
Lead in the Periodic Table
Tin is found in period 5 and group 14 or the carbon family of the periodic table.
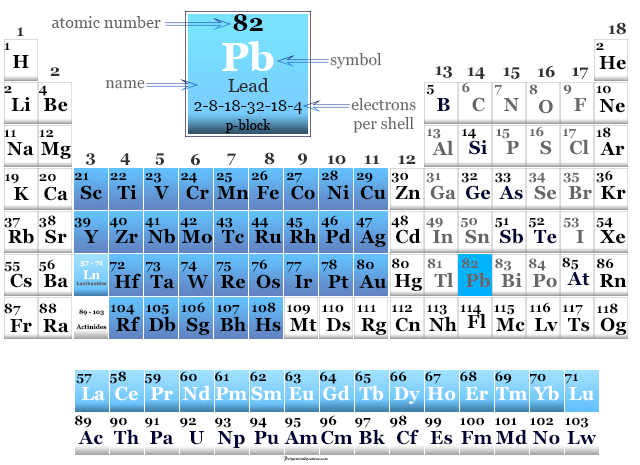
Lead is a p-block element that lies in between tin and flerovium in the periodic table.
Facts About Lead
- The chemical reactivity of group 14 elements like carbon, silicon, germanium, tin, and lead is decreasing down the group.
- Chemical potential values of Pb(II) to Pb is less than that of hydrogen but they cannot liberate hydrogen from acids.
- The finely divided lead powder is pyrophoric in nature. But the surface of the metal is inactive due to the formation of the protective oxide layer.
- It dissolves in organic acid like acetic acid or formic acid in the presence of air.
- It does not dissolve in concentrated nitric acid or sulfuric acid due to its insoluble coating.
Isotopes of Metal
Naturally, lead has four stable isotopes with mass numbers 204, 206, 207, and 208. Three of these four isotopes are found in the radioactive decay series. It is the first heaviest element whose natural isotopes are stable.
The relative abundance of these isotopes is given below in the table,
| Isotope | Relative abundance |
| 204Pb | 1.4 % |
| 206Pb | 24.1 % |
| 207Pb | 22.1 % |
| 208Pb | 52.4% |
Extraction from Galena
Galena is concentrated by the froth-flotation process. The concentrated ore is roasted by the limited supply of air to partially convert it to PbO.
Self-reduction between unchanged PbS and PbO produces lead metal.
2 PbO + PbS → 3 Pb (l) + SO2 (g)
The crude metal contains many impurities like Cu, Ag, Au, Zn, As, Sb, and Sn. It is purified by the electrolysis process.
- Impure lead is crust to rods to serve as an anode in an electrochemical cell.
- A sheet of pure metal is used as a cathode in the presence of a PbSiF6 electrolyte. The electrolyte is prepared by the action of H2SiF6 on Pb.
- Impurities are thrown down in anode mud while more electropositive metals like iron remain in the solution.
Chemical Compounds
In spite of their ns2 np2 valence shell electronic configuration, the lighter elements like C or Si of group-14 do not have any bivalent compound under ordinary conditions.
The bond energy values decrease significantly in heavier elements like Pb. Therefore, bivalent compounds are comparatively more stable at room temperature. The stability of group-14 hydrides (MH4) decreases from Ge to Pb due to decreases in M-H bond energy.
Halides
Yellow solid PbF4 is the only stable tetrahalide of Pb. It is decomposed to PbF2 and F2 on heating. It can be prepared by the action of fluorine or HF on lead compounds. Other tetrahalides are less stable and PbI4 does not exist.
The dihalides of lead are stable crystalline solids slightly soluble in cold water but much more soluble in hot water. PbCl2 and PbBr2 give photosensitive depositing electromagnetic spectrum radiation.
Lead Oxide
Pb (II) oxide or PbO2 is prepared by dissolving Pb3O4 in a dilute nitric acid solution. It is a strong oxidizing agent and decomposes to PbO at a temperature above 300 °C.
PbO is another oxide of metal that exists in two forms, litharge, and massicot.
- Litharge form is obtained by oxidizing molten Pb in the air above 600 °C in a reverberatory furnace.
- Massicot is obtained by heating Pb in the air.
PbO is basic in nature and dissolves in acids to form Pb(II) salts. It has an unusual layer structure in which four oxygen atoms form a base of the square pyramid with Pb metal at the vertex.
Lead Carbonate
Pb (II) carbonate occurs in nature as cerussite with the chemical formula PbCO3. It is precipitated from Pb(NO3)2 solution by NaHCO3 at a low temperature.
It has been used as a white pigment under the name white-Pb. However, it is now getting substituted very fast by TiO2 due to the non-toxic nature of TiO2.
Lead Acetate
Lead acetate or Pb(CH3COO)2 is a white crystalline chemical compound with sweetness in taste. It can be made by boiling Pb with acetic acid and hydrogen peroxide.
Pb is used in medicine for washing the eyes. It is also used for making other lead compounds and mordants in the dyeing process.
Uses of Lead
- It is used in plumbing in Roman civilization. Now it has been stopped due to lead poisoning.
- Worldwide production of increases due to the use of Pb-acid batteries.
- It has been used to make alloys like solder (50% Pb, 50% Sn) and type metal (70 to 80% Pb, 30 to 15%Sb). Because of their low melting point and expansion on the solidification of Pb-Sb alloy, it is called type metal.
- Lead is used for cable coverings and making pigments.
- It is also used to store corrosive liquids.
- It is used for making antiknock compounds like lead tetraethyl. However, the manufacture of lead tetraethyl is highly discouraged due to air pollution.