Acetone Chemistry
Acetone, 2-propanone, or dimethyl ketone in chemistry is a colourless and highly flammable organic compound with the chemical formula CH3COCH3. Commercially acetone can be used for the production of nail polish remover, paint remover, and varnish remover. It is also used as a solvent in the pharmaceutical industries, textile industries, and analytical chemistry laboratories for chromatography analysis. It is a pungent smelling liquid miscible with water, ethanol, and ether in all proportions. Acetone is a liquid solvent that can break down to dissolve other substances like acetylene, cellulose, acetate, nitrate, etc.
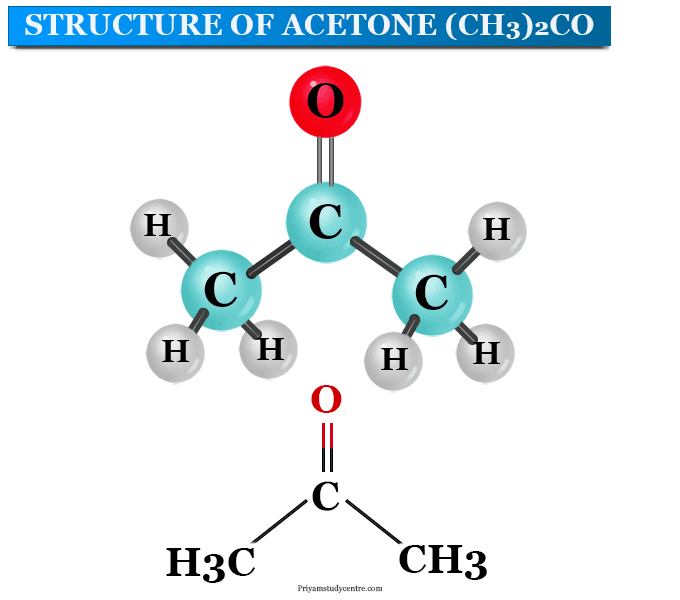
Acetone Structure
The chemical formula of acetone can be written as CH3COCH3 or C3H6O. It is the simplest ketone that contains three carbon atoms, six hydrogen atoms, and one oxygen atom.
The atoms in the acetone compound contain two methyl groups attached to the middle carbon atom or sp2 hybridized carbon atom. The middle carbon atom also contains a double bond with an oxygen atom by the formation of a carbonyl or ketone functional group. In the acetone structure, both the available valencies are attached to carbon atoms. Therefore, the keto group in acetone occurs within a chain.
Acetone Production
- Acetone can be produced industrially by catalytic dehydrogenation of isopropanol by copper or zinc oxide.
- It is also produced by the catalytic oxidation of isopropanol with a silver catalyst.
- It can be prepared by passing a mixture of propene and oxygen under pressure into an aqueous solution of palladium and cupric chlorides.
- It can also be manufactured by the oxidation of natural gas.
- The cumene phenol process is the most important process for the production of phenol and 2-propanone. It can be carried out by the oxidation of cumene to its hydroperoxide which then decomposes by dilute sulfuric acid to form phenol and 2-propanone.
Properties of Acetone
- The colourless, pleasant-smelling acetone does not polymerize but readily undergoes condensation reactions. It readily forms mesityl oxide, phorone, and diacetone alcohol.
- Ketones have a low boiling point compared to alcohol due to their ability to form intermolecular hydrogen bonding. On the other hand, carbonyl compounds have a higher boiling point than the corresponding alkanes due to the dipole-dipole interaction of carbonyl compounds.
- It forms hydrogen bonds with water. Therefore, it is soluble or partially soluble in water.
Some common properties of acetone are given below in the table,
| Properties | |
| IUPAC name | 2 propanone |
| Chemical formula | CH3COCH3 |
| Molar mass | 58.080 g mol−1 |
| Appearance | Colourless liquid |
| Odor | Pungent, irritating |
| Density | 0.7845 g/cm3 at 25 °C |
| Melting point | −94.7 °C |
| Boiling point | 56.05 °C |
| Solubility in water | Miscible |
| Dipole moment | 2.91 D |
| Heat capacity | 125.45 J mol−1K−1 |
Chemical Reactions
- Ketones like acetone do not easily oxidize. Therefore, it does not reduce Fehling’s solution or ammoniacal silver nitrate. It can be oxidized by strong oxidizing agents like acid dichromate, and nitric acid.
- It undergoes a haloform reaction. Haloform can be carried out by dissolving it in dioxan and then adding dilute sodium hydroxide and iodine in a potassium iodide solution. Warming the solution and finally adding water. Iodoform can be precipitated.
CH3COCH3 → CH3COCI3 → CH3COO− + CHI3 - It is treated with ammonia and then followed by acidification to form diacetoneamine and triacetonamine.
- It can be reduced catalytically to form alcohols. It can also be reduced by dissolving metals in an alkaline solution to form 1,2-glycols.
- In chemistry, acetone or 2-propanone condenses with chloroform in the presence of potassium hydroxide to form a chloro-hydroxy compound.
Uses of Acetone
Acetone is a common chemical compound that can be used widely in the pharmaceutical and textile industry, laboratories, and in our daily uses of domestic products. It is also used for manufacturing methyl isobutyl ketone and methyl methacrylate.
Uses as a Solvent
- Acetone is used mainly as a solvent for lacquers, cellulose, cellulose acetate, and acetylene.
- It is a good solvent for several plastics and synthetic fibers used for thinning polyester resin.
- It is one of the volatile organic compounds which used in paints and varnishes.
Uses in Medicine
- Acetone is used as a solvent in the pharmaceutical industry to ensure accurate dosage of medicine.
- It is also used for cleaning and sterilization of medical tools and equipment.
- Dermatologists can use it to treat acne present on peel dry skin.
Uses in Chemical Research
- In the chemistry laboratory, acetone is used as a polar, aprotic solvent in a variety of organic reactions.
- It is a common solvent used for rinsing laboratory equipment due to its low price and volatility.
- Acetone is used to precipitate proteins in the chemical research laboratory.
Uses in Analytical Chemistry
In analytical chemistry, it is a good pigment solvent in chromatographic research. In high-performance liquid chromatography, thin-layer chromatography, and gas chromatography techniques, we used CH3COCH3 due to its solvent character and volatility.
Domestic uses of Acetone
- It is used as an additive in makeup and skin creams.
- It is the primary ingredient of nail polish remover and superglue remover.
- It is also used for printing artifacts on 3D-printed models and ABS plastic.
- Many cleaning products use acetone for cleaning electronic gadgets and appliances.