Deviation of Real Gases from Ideal Behaviour
Ideal gas and real gas molecule can be compared by the ideal gas law or equation for different types of gases. The gases which obey ideal gas law under all conditions of temperature and pressure are called ideal gases but which do not obey ideal gas law under all conditions of temperature and pressure are called real gases. Different Gas Law formulas can be used to compare physical properties like density, molecular weight, diffusion, thermal energy, and compressibility for ideal and real gases in physics or physical chemistry. An ideal gas is a really hypothetical one because under the maintained temperature and pressure it transformed into a liquid state. However, we assumed that ideal or perfect gases molecule has no intermolecular attraction. An important single parameter called the compressibility factor is used to compare the extent of deviation of the real gases from ideal behaviour in learning chemistry or physics.
Properties of Ideal Gases
- An ideal gas can not be liquefied because the gaseous molecule has no intermolecular attraction.
- The coefficient of thermal expansion (ɑ) depends on the temperature of the gases and does not depend on nature.
- The coefficient of compressibility (β) similarly depends on the pressure and will be the same for all gases.
- When pressure is plotted against volume at a constant temperature a rectangular hyperbola curve is obtained according to Boyle’s law.
- When PV is plotted against pressure at a constant temperature a straight line parallel plot is obtained.
- If the ideal gas passes through a porous plug from higher pressure to lower pressure within the insulated enclosure, there will be no change in the specific heat or temperature. This confirms that the ideal gases have no inter-molecular attraction.
Properties of Real Gases
- Real gas could be liquefied because the gaseous molecule has the property of intermolecular attraction which helps to coalesce the molecule.
- The coefficient of thermal expansion (α) depends on the nature of the gaseous molecule.
- The coefficient of compressibility (β) was also found to depend on the nature of the gases.
- When pressure is plotted against volume a rectangular hyperbola curve is obtained only at a high temperature above the critical temperature.
- However, at a temperature below the critical temperature (TC), the molecule can be liquefied after a certain pressure depending on temperature.
- Liquid and gas can be indistinguishable properties in the critical point of the gases. When PV is plotted against pressure for real or Van der Waals gases, the Amagat curve is obtained.
- Real gases pass through porous plugs from higher pressure to lower pressure within the insulated enclosure, there occurs a change in temperature.
- Real gases have inter-molecular attraction and when expanded, the molecules have to spend more kinetic energy to overcome inter-molecular attraction. Therefore, the temperature drops down.
Coefficient of Thermal Expansion
In thermodynamics, the coefficient of thermal expansion and compressibility can be defined as,
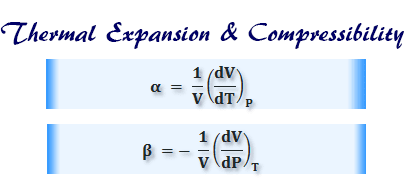
For one mole ideal gases,
PV = RT
∴ α = R/PV = 1/T
Therefore, the thermal expansion will be independent of nature and will be a function of temperature only.
For example, the coefficient of thermal expansion for hydrogen and carbon dioxide gases is 2.78 × 10−7 and 3.49 × 10−7 respectively at 0°C and 500 atm pressure. For real gases, the coefficient of thermal expansion depends on the nature of the gases.
Coefficient of Compressibility
Coefficient of compressibility for ideal gases,
β = RT/P2V = 1/P
Therefore, β should be a function of pressure only and the same for all gases. But experimentally the coefficient of compressibility has been found to be an individual property.
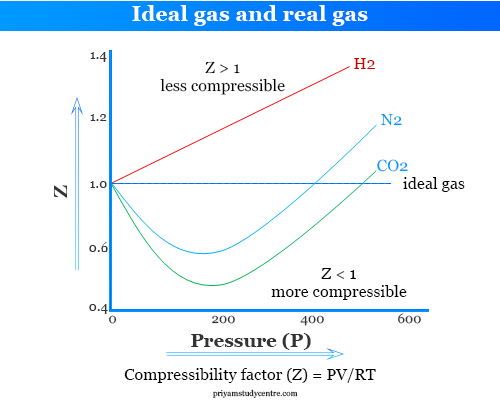
PV vs P Graph for Real Gas
When PV is plotted against P for real gases following plots are obtained. It is called the Amagat curve.
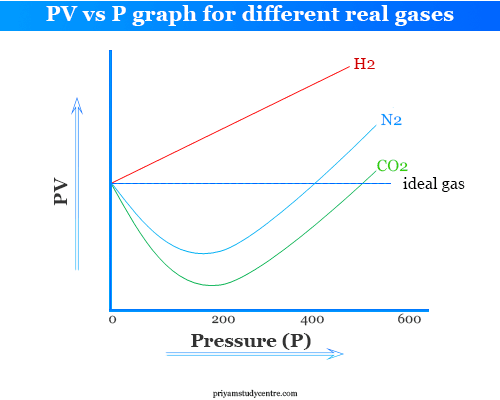
The value of PV decreases attains a minimum and then increases with the increased pressure.
- Only hydrogen and helium baffled this compression trend and the curve rose with increased pressure from the very beginning.
- Carbon dioxide can be easily liquefied and PV dips sharply below the ideal gas line in the low-pressure region.
At very low pressure (below 1 atmosphere), the plot PV-P is linear but different for different gases.
In the case of carbon dioxide, oxygen, hydrocarbon (methane, ethylene), and nitrogen, the PV values first diminish and reach the minimum, then increase.
Boyle Temperature
Every gas has a characteristic temperature where the curve PV-P runs parallel to the P-axis from zero pressure to moderate value of pressure. It means, at this temperature, the value of PV is constant for an appreciable range of pressure. This temperature is called Boyles temperature or Boyle point of the gas.
Mathematically TB can be defined as,
TB = d(PV)/dT = 0, when P → 0
| Gases | Boyles temperature |
| hydrogen (H2) | − 156 °C |
| helium (He) | − 249 °C |
| nitrogen (N2) | 59 °C |
| methane (CH4) | 224 °C |
| ammonia (NH3) | 587 °C |
Compressibility Factor Formula
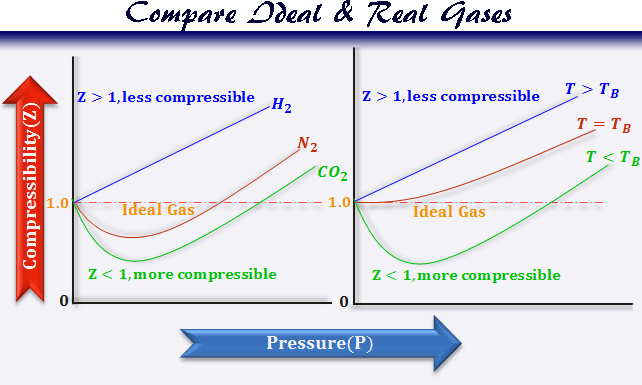
The compressibility factor can be defined as,
Z = PV/RT
where V = the molar volume
- When Z=1, there is no deviation of real gases from ideal behaviour.
- When Z ≠ 1, the departure of the unit value of Z is a measure of the extent of non-ideality.
- Z < 1, the gas is more compressible than ideal gases.
- When Z > 1, real gases are less compressible than ideal gases.