Tungsten Metal
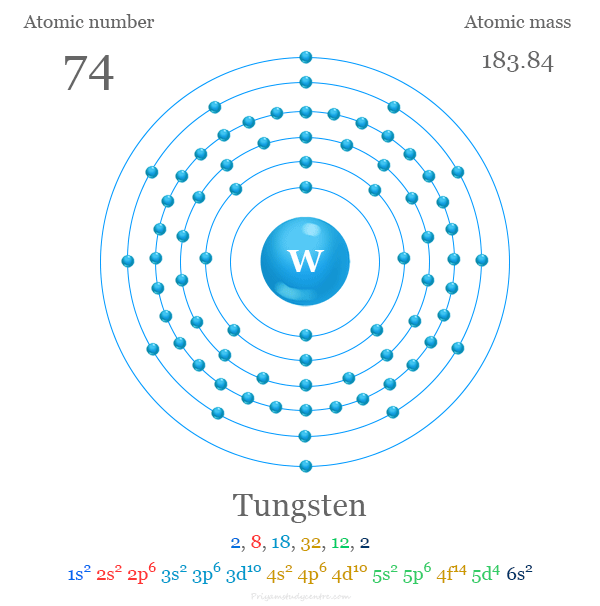
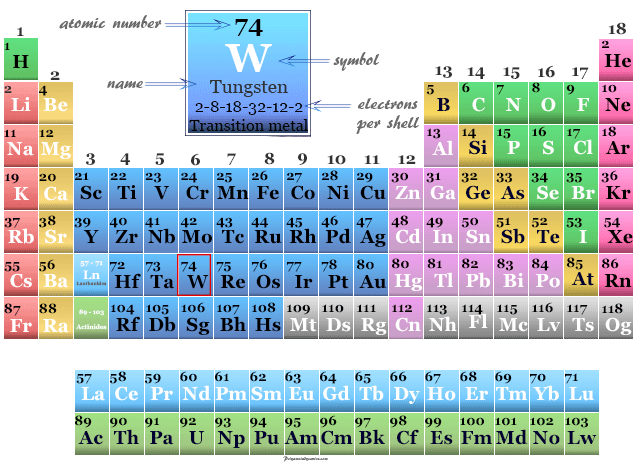
Tungsten is a chemical element or strong, silvery transition metal of Group 6 (VIB) of the periodic table with the symbol W and atomic number 74. It is used for making steel and lamp filaments due to its hardness. The symbol of tungsten is derived from the name Wolfram which is still used in some literature. The properties of tungsten and molybdenum are very similar but the chemistry of these two metals are slightly different. It has the highest melting point among all metals and second after carbon among all periodic table elements. The atomic number of tungsten is 74 and the electronic configuration [Xe] 4f14 5d4 6s2. Therefore, tungsten is placed in the d-block of the modern periodic table with group members chromium and molybdenum. It is the last metal in the third transition series in which all the d-electrons participate in metallic bonding.

Tungsten Discovery
The mineral tungsten (Swedish heavy stone CaWO4) was studied in 1751 by Cronstedt. In 1981 Carl Wilhelm Scheele discovered new oxide from this mineral.
Two years later Spanish chemists José and Fausto Elhuyar (two brothers) separated the same oxide from the mineral known as wolframite. They isolated the metal tungsten by carbon reduction reaction of charcoal. The symbol of the tungsten is derived from the name Wolfram.
Properties of Tungsten
The metal is very hard, has high melting, and is relatively unreactive due to the protective oxide layer. It forms typically metallic body-centered cubic crystal lattice like molybdenum.
The metals Cr, Mo, and W are the members of Group VI in the periodic table. The common oxidation number or state of tungsten is +6 (VI).
| Tungsten |
|||
| Symbol | W | ||
| Discovery | Juan and Fausto Elhuyar | ||
| Name derived from | Swedish word tung sten, meaning heavy stone | ||
| Periodic properties | |||
| Atomic number | 74 | ||
| Electron per shell | 2, 8, 18, 32, 12, 2 | ||
| Electronic configuration | [Xe] 4f14 5d4 6s2 | ||
| Block | d-block | ||
| Period | 6 | ||
| Group | 6 | ||
| Atomic weight | 183.84 | ||
| Common isotopes | 182W, 184W, 186W | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 3695 K (3422 °C, 6192 °F) | ||
| Boiling point | 6203 K (5930 °C, 10706 °F) | ||
| Density | 19.3 g/cm3 | ||
| Molar heat capacity | 24.27 J mol−1 K−1 | ||
| Electrical resistivity | 52.80 nΩ·m | ||
| Atomic radius | 2.18 Å | ||
| Covalent radius | 1.50 Å | ||
| Crystal structure | body-centered cubic (bcc) | ||
| Chemical properties |
|||
| Common oxidation number | 6, 5, 4, 3, 2, 0 | ||
| Electronegativity | 2.36 (Pauling scale) | ||
| Electron affinity | 78.757 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | |
| 770 | 1700 | ||
| CAS number | 7440-33-7 | ||
Where is Tungsten Found?
Tungsten is found in nature mostly as wolframite, Fe(Mn)WO4, and scheelite or calcium tungstate, CaWO4.
The main reserve places of metal W are China, the United States, South Korea, Bolivia, Portugal, and Russia.
Isotopes of Tungsten
The metal tungsten has four natural isotopes (182W, 183W, 184W, and 186W) and one radioactive isotope (180W). However, the radioactive isotope of the metal has very long lives.
All five isotopes radioactive decay to form hafnium by nuclear reaction. However, another 30 radioactive isotopes of metal are observed. These can be obtained by the different types of nuclear reactions.
Production of Tungsten Powder from Wolframite
- The concentrated ore of tungsten like wolframite is roasted in the air with sodium carbonate or fused sodium hydroxide (NaOH).
- The sodium tungstic acid is extracted and treated with water. The precipitated tungstic acid is heated to obtain WO3.
- Pure W metal is obtained during the reduction of metal oxide with zinc, aluminum, or hydrogen.
- The metals molybdenum and tungsten have very high melting points and they are obtained initially in the form of powder. Tungsten is generally converted to a massive state by compression under hydrogen at high temperatures.
Chemical Compounds
The chemistry of tungsten is close to molybdenum. The most common and stable oxidation state of the metal is +6 but the +5 and +4 states are also stable.
Strong reducing properties of the metal are not met before the +3 and +2 states. The oxides, fluorides, and oxoacids are formed in different oxidation states.
Oxides
The yellow WO3 is the end product of heating other compounds of tungsten in the air. It is insoluble in acids. WO3, 2H2O is formed in strongly acidic media. It is called tungstic acid but it is not a hydrated acid.
A few lower oxides like WO2 were obtained during the reduction of trioxide with hydrogen. The acidic nature of the oxides decreases with decreasing oxidation state.
Halides
The hexahalides of the metal are obtained by the direct combination. The colorless fluoride like WF6 is more stable. It is quite reactive and readily reacts with oxygen and moisture to form oxohalide and HF. WBr6 is unstable and decomposes on gentle warming. The oxohalides of the metal are two types
- WOX4 (X = F, Cl, Br): They are volatile, solid covalent compounds, hydrolyzed to give WO3.
- WO2X2 (X = F, Cl, Br, I): The yellow colour WO2Cl2 decomposes above 200 °C to form WO3 and red WOCl4.
The petahalides of tungsten metal are also known. The yellow halide like WF5 is formed by the reduction of WF6 at 500 to 800 °C. Above 30 °C the yellow solid disproportionate reaction between WF4 and WF6.
The tetrafluoride of the metal is formed by the reduction of hexafluoride with benzene or other hydrocarbons. It is a nonvolatile red-brown chemical compound.
Halide Clusters
The lower oxidation state of tungsten is unstable and strongly reducing in nature. Therefore, the metal only forms WCl3 and WBr3 in a +3 oxidation state but W (II) is completely unstable.
The complex halide of W (II) is W6X12 type (where X = chlorine, bromine, iodine). It forms cluster compounds like (W6X8)+4 X4−. The oxidation state zero is also observed in the carbonyl complexes like W(CO)6.
Analytical Reactions
Hydrochloric acid addition to a tungstate, hydrated tungstic acid is precipitated. On heating, the white precipice of tungstic acid turns yellow. It may be confirmed by heating the precipitate with Zn and HCl or SnCl2 when a blue precipitate is formed.
Tungstates are precipitated as benzidine tungstates from a dilute 0.1N sulfuric acid. The precipitate is ignited to WO3 and weighed. At pH scale of 7 to 8 tungstates can be precipitated as BaWO4.
What is Tungsten Used For?
- Pure tungsten metal is used in making filaments of old-style electric lamps or bulbs and anticathodes in X-ray tubes.
- Tungsten is an important alloying metal for high-speed tools due to its hardness. It forms several very hard alloys such as satellite (cobalt, chromium, and tungsten) and Widia metal (WC + 10 percent copper).
- The compound W (IV) sulfide is used generally in high-temperature lubricants and calcium/magnesium tungstates are used widely in fluorescent lighting.
- The oxide form like WO3 is used as a chemical catalyst in power plants. It generally converts nitrogen oxide to nitrogen and water in the presence of ammonia.
- Due to its hardness tungsten carbide (WC) is an important metal carbide for the mining and petroleum industries. It is used for making different types of high-speed cutting tools. It can be prepared by mixing tungsten powder and carbon powder at 2200 °C.
Biological Role of Tungsten
Tungsten is the only element in the third transition series that functions in the biological system. However, a protein containing the metal is rare and found generally in thermophilic anaerobes.
Tungsten also occurs in several enzymes with molybdenum. The mode of the biological function of the tungsten metal in enzymes involves oxo-transfer of W (VI) to the substrate.