Magnesium Element
Magnesium is a chemical element, alkaline earth metal of Group 2 (IIA) of the periodic table with the symbol Mg and atomic number 12. It is an essential metal that controls hundreds of chemical reactions in our body for good health. It is used widely in lightweight construction. In the hexagonal closed-packed crystal lattice, magnesium is silvery-white, lustrous, and relatively soft metal. The chemistry of magnesium is marked by the small size and consequently strong electric polarization of Mg+2 ion. It is much harder than alkali metals due to the use of two electrons for chemical bonding. The ionization energy is also much more than that of beryllium. This is mainly because the size of Mg+2 is much higher than that of Be+2.

Properties of Magnesium
The third ionization energy of the Mg and other alkaline earth metals is much higher due to the closed-shell electronic configuration like the M+2 ion.
| Magnesium | |||
| Symbol | Mg | ||
| Discovery | Joseph Black in 1755 | ||
| Name derived from | A district of Eastern Thessaly in Greece | ||
| Common isotope | 12Mg24 | ||
| Oxidation number or state | +2 | ||
| CAS number | 7439-95-4 | ||
| Periodic properties | |||
| Atomic number | 12 | ||
| Atomic weight | 24.305 | ||
| Electron per cell | 2, 8, 2 | ||
| Electronic Configuration | [Ne] 3s2 | ||
| Block | s-block | ||
| Group | 2 | ||
| Period | 3 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 923 K (650 °C, 1202 °F) | ||
| Boiling point | 1363 K (1091 °C, 1994 °F) | ||
| Molar heat capacity | 24.869 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 1.74 g/cm3 | ||
| Electrical resistivity | 43.9 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 1.73 Å | ||
| Covalent radius | 1.40 Å | ||
| Electronegativity | 1.31 (Pauling scale) | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 737.75 | 1450.68 | 7732.69 | |
Magnesium in the Periodic Table
It has a 3s2 electronic configuration over a noble gas core. This electronic configuration of the magnesium metal leads to its inclusion in group-2 (IIA) with the alkaline earth family of the periodic table. Commonly, it loses two s2 electrons to show a +2 oxidation number or state.
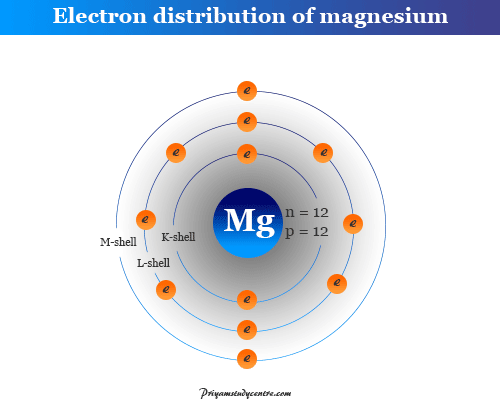
Atomic Structure of Magnesium
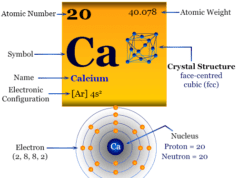
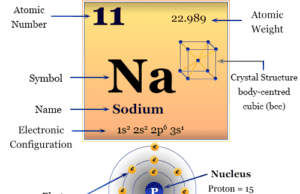
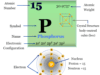
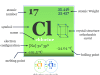
The atomic number of magnesium is 12 and the mass number is 24. Therefore, it contains 12 electrons, 12 protons, and 12 neutrons inside the nucleus. The distribution of these subatomic particles is given below the picture,
Where is Magnesium Found?
Magnesium is a highly abundant metal that is found in about 2.1 percent of the earth’s crust. It occurs principally as sedimentary rocks like dolomite (MgCO3 + CaCO3) and other evaporated forms like carnalite (KCl, MgCl2, 6H2O).
A huge quantity of magnesium (0.13 percent) is found in sea water. The majority supply of metal now comes from the sea water. A large amount of metal is found in the mantle of the earth (olivine).
Magnesium in Human Body
Magnesium is the eleventh most abundant element by mass in the human body which controls the biological reactions of different types of enzymes.
The chlorophyll in the plants contains Mg+2 ions. Its compounds are widely used in medicine, antacids, and stabilize abnormal nerve excitation or blood circulation.
Production Process
It is produced either by electrolysis or silicothermal reduction.
In the electrolytic method, the fused anhydrous mixture of (MgCl2 + CaCl2 + NaCl) is electrolyzed at 750 °C using an iron cathode and graphite anode in an inert atmosphere.
In the silicothermal process or Pidgeon process, magnesium oxide or calcinated dolomite (CaO, MgO) is heated with ferrosilicon at about 1150°C in an alloy steel reactor. A high vacuum is maintained to distill out Mg formed by the production process.
Facts About Magnesium
The properties of magnesium differ from the other member of the group in their behavior toward liquid ammonia.
- Alkali metals, Ca, Sr, and Ba dissolve in liquid ammonia to form deep black solutions.
- But Be and Mg do not readily dissolve in liquid ammonia. On prolonged boiling with liquid ammonia, it evolved hydrogen to form a blue solution.
Chemical Compounds
Magnesium Hydride
The hydride of metal is obtained by the direct combination of magnesium with hydrogen at 200 to 400 °C.
Hydride like CaH2, SrH2, and BaH2 is formed by ionic bonding but BeH2 is formed by covalent bonding. Therefore, MgH2 has the properties to form an intermediate between those ionic and covalent bonds.
Magnesium Oxide and Hydroxide
An oxide like MgO is an ionic compound with a high melting point. The oxide reacts in water to form hydroxide.
The basic properties of alkaline earth metal hydroxide increase from beryllium to barium. Beryllium hydroxide is amphoteric but all the other hydroxides are basic in nature.
Halides
It combines with halogens at a suitable temperature to form dihalide. These are also obtained by reacting the halogen hydracid with metal or its oxide.
However, anhydrous magnesium halide cannot be obtained by simply heating the aqueous solution due to hydrolysis. The reaction of the halogen on a mixture of metal oxide and carbon provides a better method. Anhydrous magnesium halides dissolve in certain organic solvents like alcohol, ether, and ketone.
Organometallic Compounds
Magnesium dialkyls and diaryls may be made by the reaction of LiR or LiAr on Grignard reagent in the presence of pyridine at 100°C.
Mg + H2 + 2 C2H4 → MgEt2
Grignard reagents (MgRX) are the mostly used organometallic compounds of the metal. It is made by the slow addition of the alkyl or aryl halide to a stirred suspension of magnesium metal in ether or other solvents. It has wide applications in synthetic organic or hydrocarbon chemistry.
What is Magnesium Used For?
- Magnesium is the third most commonly used metal after iron and aluminum and its alloys are widely used in lightweight construction like automobile and aircraft parts. Alloying is generally made with 2 to 9 percent aluminum, 1 to 3 percent zinc, and 0.1 to 1 percent manganese.
- More than 3,00,000 tonnes of metal are produced annually over the world for the manufacture of different types of structural material.
- It is used as a good and useful reducing agent.
- Magnesium turnings are also used to reduce UF4 in graphite-lined steel reactors to produce uranium metal.
- It is an important mineral that controls hundreds of biological reactions for the good health of our life.
- Due to its low density and good mechanical and electrical properties, magnesium is used for the production of mobile phones, computers (laptop and tablet), cameras, and other electronic components.
Function of Magnesium in the Body
Magnesium is an essential element for the human body to control different types of biological functions.
60 percent of the total Mg of the adult human body is distributed in the skeleton while the remaining is present inside the cells. The Mg+2 ion in the cell next most important cation after K+.
The small doubly charged cation has a high charge/size ratio. It is strongly hydrated as [Mg(H2O)6]+2. Therefore, the hydrated cation binds the ROPO3H group of nucleotides (ATP, ADP) and polynucleotides (DNA, RNA).
The ion may form strong complexes with oxygen atoms of various phosphate groups. Such complexes are the source of energy for the human body when necessary.
Nerve impulse transmission, muscle construction, and metabolism of carbohydrates are also influenced by magnesium (Mg+2) ions and nucleic acids.