Alkene or Olefin in Organic Chemistry
Alkenes names as olefins like ethylene, propylene, butylene, etc are unsaturated hydrocarbon containing at least one double or olefinic chemical bond having different types of geometric structure and structural isomerism. The common molecular formula of alkenes or olefins is CnH2n, where n = 1, 2, 3, etc. Alkene or olefin molecules are named by different methods like common olefin material, substituted names, and IUPAC nomenclature of organic compounds in chemistry.
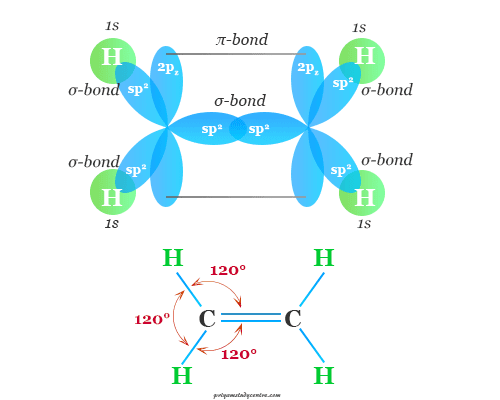
The double bond in alkene is called the olefinic bond or ethylenic bond. The electronic configuration of double-bonded carbon in the excited state, 1s2 2s1 2px1 2py1 2pz1. The 2s, 2px, and 2py orbitals hybridized to form three equivalent sp2 hybrid orbitals, and the remaining 2pz-orbital are undisturbed.
sp3 hybrid orbitals form three sigma bonds with the hydrogen atom or alkyl group present in alkenes or olefins molecule and reaming 2pz-orbital form pi-bond or olefinic bond with the neighboring carbon atom.
In learning chemistry, the 2pz-electrons are known as pi-electrons, mobile electrons, or unsaturation electron particles because they form the pi bonding in alkenes or olefins molecules.
Alkenes or Olefins Molecule
The double bond of the alkene molecule is known as ‘olefinic’ bond or ‘ethylenic bond’. But the organic naming of olefin molecules is defined from ethylene which is known ‘olefiant gas’ or oil-forming gas of our environment.
Since alkenes form an oily liquid when oxidation with chlorine or bromine water, or dilute base solution of potassium permanganate. Therefore, alkenes are named as olefins. The double bond presence in alkenes or olefins is readily detected by electromagnetic spectrum (infrared) with C=C stretching frequency 1680 to 1620 cm-1.
The original name of the homologous series of alkenes was olefin. But later decided to reserve the suffix – ine for basic substances only. Since the naming of olefins compounds gained and use widely for alkenes. For example, some common alkenes or olefin molecules are,
- Ethylene (CH2=CH2)
- Propylene (CH3-CH=CH2)
- Butylene (CH3-CH2-CH=CH2), etc
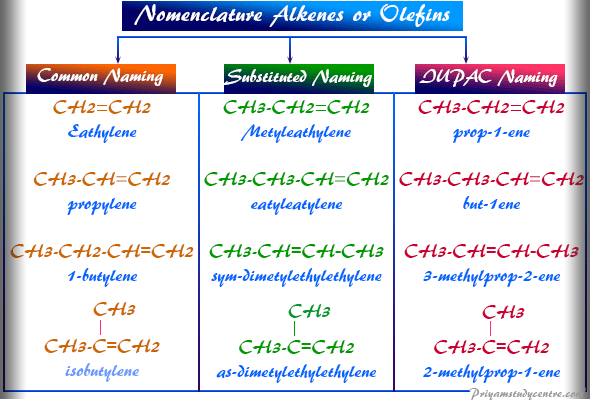
Common Naming of Alkene or Olefin
Common naming of alkenes or olefins compounds given from the corresponding alkane molecules and named according to the following rule
- The total number of carbon atoms counted in the olefin and the name of the corresponding alkane.
- Changing the name of the corresponding alkane, the suffix -ane of the latter into – ylene.
- The defined position of the olefin bond is indicated by numbers 1, 2, 3, 4…., or Greek letters like alpha, beta, gamma, delta,…
- The locants of the double-bonded carbon atom are placed before the name of the olefin.
- A hyphen has been written between the locants and the name.
The locants are used only to name alkenes or olefins containing more than three carbon atoms. Therefore, the alkenes with low molecular weight only have common names. Examples of such naming of alkene molecules are,
- ethylene (CH2=CH2CH3)
- 1 – butylene or α – butylene (CH2CH2CH=CH2)
- 2 -pentylene or β – pentylene (CH3CH=CHCH2CH3), etc
Substituted Naming of Alkenes
In this method, ethylene is considered the parent substance. Hence the higher member is the derivatives of ethylene. When we name mono-substituted alkenes or olefins molecules no difficulty arises. However, the disubstituted derivative of ethylene is named as an asymmetrical and symmetrical hydrocarbon.
- When the groups are attached to the same carbon atom of the alkenes or olefins nomenclature as the asymmetrical organic compounds.
- But when the groups attached to the different carbon atom of the alkene or olefin nomenclature as the symmetrical. For example, ethyl ethylene (CH3CH2CH=CH2), as-dimethyl ethylene (CH3(H3C)C = CH2), sym-dimethyl ethylene (CH3CH = CHCH3).
IUPAC Nomenclature Alkenes or Olefins
- According to the IUPAC nomenclature of alkenes or olefins, the suffix name of the olefins is ene. Therefore, the series becomes the alkene series.
- Find the longest carbon chain structure containing the olefinic bond name as the parent alkene in the IUPAC nomenclature.
- The position of the double bond and side chains is indicated by numbers or locants. The lowest number of locants was given to the double bond and placed before the suffix.
- Hence the name of alkenes or olefins is obtained by changing the suffix – ane to ene. For example, alkanes like methane and ethane into alkenes methene and ethene.
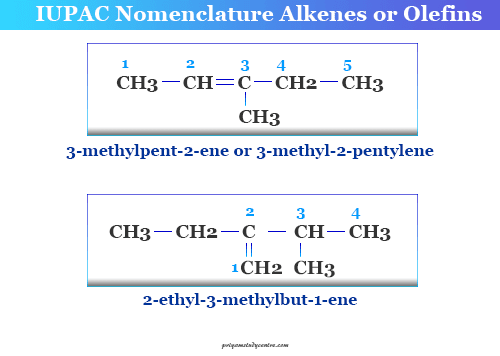
For example, the IUPAC name alkene containing a double bond and a side chain in the structure, CH3-CH=C(CH3)-CH2-CH3 is 3-methylpent-2-ene. The double bond in alkene or olefin compound is regarded as a functional group like the alcohol or alcoholic, carboxylic acid, carbonyl group, etc.
Structural Isomerism of Butylene and Pentylene
An organic compound that has the same molecular weight but differs in its structure is said to the structural isomerism. From this definition, alkene or olefin like ethylene, propylene has no structural isomer. However, greater than three carbon atoms show structural isomerism.
- For example alkenes or olefins like butylene with the molecular formula, C4H8 have three structural isomers.
- However, olefins like pentylene with molecular formula C5H10 have five structural isomers.
Now take each one in turn and introduce one double bond in isomeric pentene. Starting at the least substituted end and shifting the double bond inwards. Therefore, the isomeric pentylene shows five structural isomers. The IUPAC nomenclature of these alkenes or olefins,
- pent-1-ene
- pent-2-ene
- 2-methyibut-1-ene
- 3-methyibut-1-ene
- 2-methyibut-2-ene
Isomerism of Alkenes or Olefins Molecule
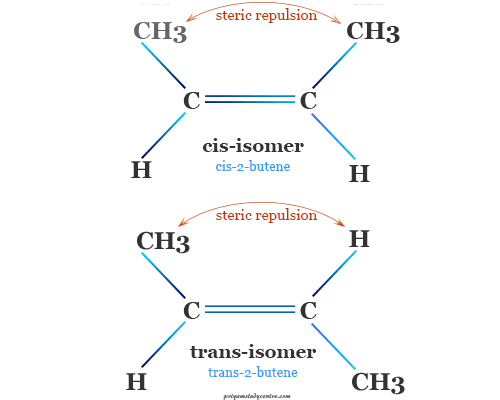
The stereoisomer’s dimensions in organic molecules like alkenes or olefins depend on four important factors which are dipole moment or electric polarization, bond opposite strain, or polarity of covalent bonds in alkene, steric strain, and bond angle strain.
Stereoisomers have the same chemical structure but different configurations. There are two types of stereoisomers
- Optical isomerism
- Geometrical isomerism
Alkenes or olefins are shown only geometric cis, and trans isomers because they are not optically active molecules.