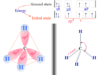
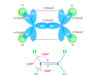
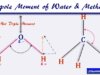
Physical Properties of Alkenes
Physical properties of alkenes and alkanes in organic chemistry are very similar. Alkenes are unsaturated hydrocarbons but alkanes are saturated hydrocarbons. Due to the presence of a double bond or olefinic bond, alkenes have participated in a wide number of chemical reactions. They are colourless and odourless in nature but ethylene faintly sweet odour. The alkenes are insoluble in water due to their nonpolar properties. The stability of alkenes depends mostly on the heat of combustion or hydrogenation. The gaseous, solid, and liquid forms of alkanes are found in nature and it depends on the number of carbon atoms present in their structure. Alkenes containing two to four carbon atoms are gases but five to seventeen are liquid. However, greater than eighteen are crystalline solids at room temperature.
Why Alkenes are Called Olefins?
The name of unsaturated hydrocarbon alkene comes from ethylene. Ethylene is called oil-forming or olefiant gas and forms an oily liquid when treated with chlorine or bromine. Therefore, these classes of organic compounds are called olefinic compounds.
Stability of Alkenes
Heat of Hydrogenation and Stability of Alkenes
To measure the stability of an alkene, we measure the heat of combustion or hydrogenation. The heat of hydrogenation is an exothermic reaction. Numerically, alkenes that have a smaller value of ΔH are more stable.
The heat of combustion only compares the stability of different alkenes which produces the same alkanes on hydrogenation.
| Alkenes | The heat of hydrogenation (kJ mol−1) |
| ethylene | −137.2 |
| n-propylene | −125.9 |
| n-butylene | −126.8 |
| cis-2-butylene | −119.7 |
| trans-2-butene | −115.5 |
| isobutylene | −118.8 |
Stability of Alkenes by Steric Effect
Enthalpy of formation of alkenes not purely addictive properties. Therefore, the stability of alkenes depends on the steric effect or hyperconjugation.
Three isomers of butylene give the n-butane on reduction. The order of stability of these butylene isomers is as follows:
trans-2-butene > cis-2-butene > 1-butene
In cis-2-butene, the two methyl groups in the cis isomer are closer together than in the trans isomer. Therefore, cis-2-butene experiences greater steric repulsion. Nence the cis-isomer has a greater strain than the trans-isomer.
Due to steric destabilization, the stability order of cis and trans butylenes is,
trans-2-butene > cis-2-butene
Stability of Alkenes by Hyperconjugation
A strict effect destabilizes organic molecules but hyperconjugation stabilizes. Among 1-butene and 2-butene, 1-butene has a less number hyperconjugation structure. Therefore, 1-butene is less stable than 2-butene.
Since trans-2-butene is the most stable isomer, it follows that hyperconjugation has a greater stabilizing effect than steric repulsion.
Heat of Combustion and Stability of Alkenes
The stability of alkenes may compared to the heat of combustion determination. The heat of combustion for n-butylenes is given below in the table,
| Alkenes | The heat of combustion (kJ mol−1) |
| n-butylene | −2719 |
| cis-2-butylene | −2719 |
| trans-2-butene | −2707 |
| isobutylene | −2703 |
The above four butylenes give the same product on combustion. Therefore, the stability order of the above four butylenes is
isobutylene > trans-2-butylene > cis-2-butylene > 1-butylene
Question: Arrange Me2C=CH2, cis-MeCH=CHMe, trans-MeCH=CHMe, MeCH2CH=CH2, these alkenes in order of increasing stability.
Answer: The increasing stability order of the above alkenes is,
MeCH2CH=CH2 < cis-MeCH=CHMe < trans-MeCH=CHMe < Me2C=CH2
The order of stability of general alkenes,
R2C=CR2 > R2C=CHR > R2C=CH2 ~ RCH=CHR > RCH=CH2 > CH2=CH2
Where R = alkyl or a methyl group
Chemical Properties of Alkenes
Owing to the presence of a double bond, the alkenes undergo a large number of addition reactions but under special conditions, they also undergo substitution properties.
The high reactivity of alkenes is due to a double bond or olefinic chemical bond that contains π- electrons. Addition reactivity occurs due to a change of trigonal arrangement to the tetrahedral arrangement or formation of saturated hydrocarbon.
Geometrical Isomerism
Isomers are molecules that have the same structure but differ in their spatial arrangement and are called stereoisomers. There are two types, optical and geometrical isomerism.
Many alkenes have one double bond and exist in two forms, cis and trans-form. Many physical and chemical properties of cis and trans-alkenes differ widely. Van’t Hoff suggested that if we assume there is no free rotation about a double bond or functional group, two structural arrangements are possible for the molecules.
Why there is no free rotation around a double bond?
If we assume two unsaturated carbon atoms are sp3 hybridized with excited-state electronic configuration,
1s2 2s1 2px1 2py1 2pz1
They are joined by two banana bonds with a maximum overlap of orbitals. To decrease such overlap, bond energy must be supplied to break the chemical bond. This fact restricted the free rotation of double bonds.
If we assume sp2 hybridization of two unsaturated carbon atoms, the double-bonded pi-orbitals are maximum overlap. Such a fact causes to resistance the rotation of the double bond.
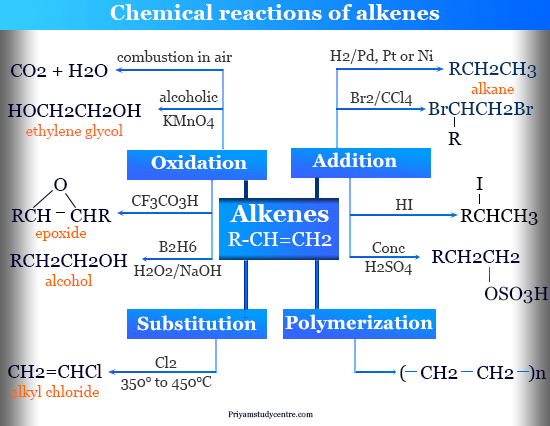
Chemical Reactions of Alkenes
Due to the presence of π-bond, alkenes participate in a large number of organic reactions. Some important chemical reactions are:
- Hydrogenation of alkenes
- Combustion of alkenes
- Addition reactions of alkenes
- Addition of hydrogen halides
- Hydration of alkenes
- Hydroxylation of alkenes
- Ozonolysis of alkenes
- Prileschaiev’s Reaction
- Hydroboration oxidation
- Polymerization reaction
Hydrogenation of Alkenes
Alkenes are readily hydrogenated under pressure in the presence of a chemical catalyst.
C2H4 (ethylene) + H2 → C2H6 (ethane)
For the hydrogenation of alkenes at room temperature, we used finely divided platinum or palladium, or Raney nickel. Nickel can also be used between 200 °C to 300 °C.
When the alkenes are the type of R2C = CR2 or R2C = CHR, the hydrogenation under the above conditions is difficult. For hydrogenation of such types of alkenes, we used sodium in ethanol or lithium aluminum hydride.
R2C = CHR → R2CHCH2R
Combustion of Alkenes
Alkenes are flammable substances. They burn in air or oxygen to form a luminous smoky flame. Generally, it produces carbon dioxide and water.
2 CnH2n + 3 H2O → 2n CO2 + 2n H2O + ΔH
CH2=CH2 + 3 O2 → 2 CO2 + 2 H2O + ΔH
Addition Reactions of Alkenes
Alkene has properties to form addition compounds with chlorine or bromine. For example, ethylene adds bromine to form ethylene dibromide.
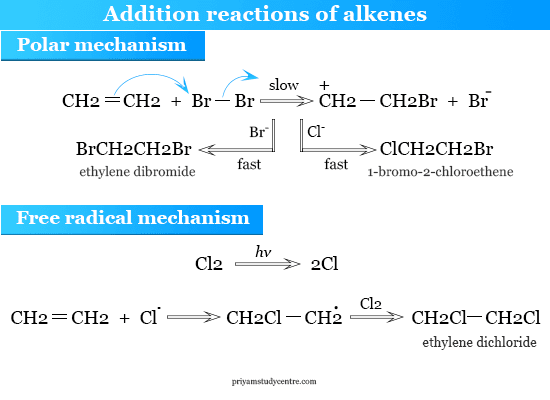
Halogen addition or halogenation of alkenes takes place by
- Heterolytic (polar mechanism)
- Free-radical mechanism
Polar Mechanism
Halogen addition radially occurs in the solution. In the absence of light or peroxides (hydrogen peroxide), it is generally catalyzed by inorganic halides like aluminum chloride. These facts lead to the conclusion that the reaction occurs by a polar mechanism.
Free Radical Mechanism
The free radical mechanism has generally been accepted due to the properties of the halogen addition to alkenes in the absence of light.
Cl2 → 2Cl·
CH2=CH2 + Cl· → CH2Cl−CH2·
CH2Cl−CH2· + Cl2 → CH2Cl−CH2Cl + Cl·
Stewart showed that the addition of chlorine to ethylene is accelerated by light. This suggested the free radical mechanism of alkenes.
Addition of Hydrogen Halides
Ethylene adds hydrogen bromide to form ethylene bromide. The order of reactivity of the halogen acids is,
HI > HBr > HCl > HF
It is also the order of acid strength.
The conditions for the addition are similar to those for halogens. The addition of hydrogen fluoride occurs under pressure.
In the case of unsymmetrical alkenes, the addition of the halogen acid takes place in two different ways.
- Propylene might be added to hydrogen iodide to form propyl iodide.
CH2CH=CH2 + HI → CH3CH2CH2I - However, it might be added to hydrogen iodide to form isopropyl iodide.
CH3CH=CH2 + HI → CH3CHICH3
Markovnikov studied many halogen addition reactions of this type and formulated an empirical rule called the Markovnikov rule.
Markovnikov Rule
According to Markovnikov, the negative part of the addendum adds to the carbon atom that is joined to the least number of hydrogen atom. Therefore, propene reacts with halogen acid to form isopropyl halide.
CH3CH=CH2 + HI → CH3CHICH3
Mechanism of Markovnikov’s rule
Markovnikov’s rule is empirical but may be explained theoretically on the basis of the polar mechanism. The addition of halogen acid is an electrophilic reaction. Therefore, the proton adds fast, followed by a halide ion.
CH3−CH=CH2 + H−I → CH3CH+CH3
CH3CH+CH3 + I− → CH3CHICH3
The addition is predominantly in the trans-position. It can be explained by the formation of a bridge carbonium ion.
When we consider propylene, the methyl group has a +I effect. The π electron is displaced towards the terminal carbon atom and it acquires a negative charge. Therefore, the proton is added to the carbon fastest from the methyl group, and the halide ion is then added to the carbonium ion.
Alternatively, Markovnikov’s rule can be explained in terms of the stabilities of carbonium ions. The stability of primary, secondary, and tertiary carbonium ions are
tertiary (Me3C+) > secondary (Me2CH+) > primary (MeCH2+)
It can be explained by hyperconjugation or no bond resonance. The number of hydrogen atoms available in primary, secondary, and tertiary carbonium ions for no bond resonance is 3, 6, and 9 respectively. Therefore, tertiary carbonium ion is the most stable.
Hydration of Alkenes
Alkenes may be hydrated to alcohol by absorption in concentrated sulfuric acid followed by hydrolysis.
CH2=CH2 + H+ → CH2CH2+
CH3CH2+ + H2O → CH3CH2OH
Ethanol is manufactured during the direct hydration of ethylene with steam under pressure in the presence of a phosphoric acid catalyst.
Hydroxylation of Alkenes
Hydroxylation of alkenes is an oxidation reaction where alkenes are mostly hydroxylated to form dihydroxy compounds or glycols. The hydroxylation reaction is carried out generally by potassium permanganate solution, hydrogen peroxide in glacial acetic acid, or osmium tetroxide.
- When a cold dilute alkaline permanganate solution is used, ethylene is converted into ethylene glycol.
CH2=CH2 + H2O + [O] → HOCH2CH2OH - Oleic acid is converted to 9, 10-dihydroxy stearic acid by 90 percent hydrogen peroxide in glacial acetic acid or formic acid.
- Osmium tetroxide is readily added to an ethyleneic double bond at room temperature to form cyclic compounds (osmium esters). These cyclic compounds can be refluxed with aqueous ethanolic sodium hydrogen sulfate to form 1,2-glycols or cis-glycols.
Prileschaiev’s Reaction
Prileschaiev’s reaction is an organic reaction where alkenes can be converted into epoxide (alkene oxide) using peracids such as perbenzoic acid, C6H5COO2H, monoperphthalic acid, p-nitoperbenzoic acid, etc. For example, ethylene can be converted to ethylene oxide in the presence of peracids.
Ozonolysis of Alkenes
Ozone is a highly reactive allotrope of oxygen. Therefore, alkenes are added readily to ozone to form ozonides. Commonly, ozonide is prepared by dissolving olefinic compounds in a solvent that can be unaffected by ozone molecules. Generally, chloroform, carbon tetrachloride, glacial acetic acid, and light petrol are used as a solvent in the ozonolysis reaction.
The oxidation or reduction of ozonide by a specific reagent gives corresponding aldehydes, ketones, carboxylic acids, or alcohols.
- Ozonide can be oxidized to form acids and ketones by silver oxide, hydrogen peroxide, or peracids.
- Reduction of ozonide with zinc-acid, hydrogen-rany nickel, or triphenylphosphine gives mostly aldehydes and ketones.
- When reduction is carried out by lithium aluminum hydride, or sodium borohydrate, the productions are the corresponding alcohols of carbonyl compounds.
Hydroboration Oxidation
Hydroboration oxidation is an organic reaction where alkenes can be converted naturally into primary alcohols. For example, propylene reacts readily with diborane in ether at room temperature to form tripropylborane. The oxidation of tripropylborane by alkaline hydrogen peroxide gives primary alcohol 1-propanol.
3 CH3CH=CH2 + BH3 → (CH3CH2CH2−)3B
(CH3CH2CH2−)3B + H2O2 → CH3CH2CH2OH
Polymerization Reaction
Generally, when two compounds (inorganic or organic) have the same empirical formula but differ in molecular weight, the more complicated compound is called a polymer of the simpler one. Polymers are classified into the following types:
- Biopolymers: Rubber, proteins, nucleic acids, starch, gum, resin, silk, wool, etc.
- Semi-synthetic polymers: Nitrocellulose, cellulose acetate.
- Synthetic polymers: Nylon, PVC, Teflon, bakelite, perspex, etc.
The term polymerization is used to describe the process where a single substance (monomer) can be converted to products having the same empirical formula but a different formula.
For example, when liquid ethylene molecules are heated to 150 to 200 °C in the presence of peroxide or a Lewis acid, a large number of ethylene molecules polymerize to form a log chain molecule of polyethylene.
n CH2=CH2 → [−CH2−CH2−]n
The addition polymerization reaction of olefinic compounds (alkenes) can be accelerated in the presence of ionic-type catalysts or radical-type catalysts. Ionic catalysts commonly are Lewis acids such as H2SO4, HF, AlCl3, BF3, etc. Organic and inorganic peroxides and peracids are the common types of free radical catalysts that are used mostly in the polymerization of alkenes.