Germanium
Germanium Element
Germanium is a chemical element or hard, lustrous, grayish white metalloid of group-14 of the periodic table with the symbol Ge and atomic...
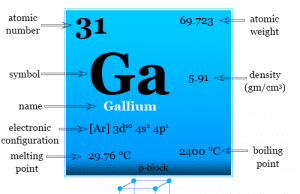
Gallium Element
Gallium Element
Gallium is a p-block chemical element or soft, silvery metal of group-13 of the periodic table with atomic number 31 and symbol Ga....
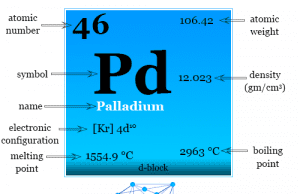
Palladium
Palladium Element
Palladium is a chemical element or silvery white, lustrous, malleable, high density metal of group-10 of the periodic table with atomic number 46...
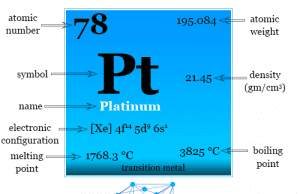
Platinum
Platinum Metal
Platinum is a chemical element or silvery white, lustrous, malleable, high density metal of group-10 of the periodic table with atomic number 78...
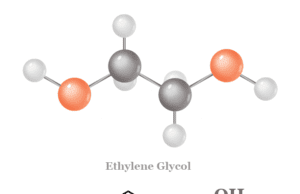
Ethylene Glycol
Chemical Formula for Ethylene Glycol
Ethylene glycol or ethane-1,2-diol is a colourless viscous liquid with the chemical formula (CH2OH)2. It is missable in all proportions...
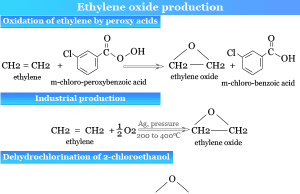
Ethylene Oxide
Ethylene Oxide Gas
Ethylene oxide gas (EtO) is a cyclic ether or simple organic epoxide compound with the chemical formula C2H4O. French chemist Charles-Adolphe Wurtz...
Ethylene
Ethylene Gas
Ethylene is a simple organic compound or unsaturated hydrocarbon that contains a double bond with the molecular formula C2H4 or CH2=CH2. It may be...
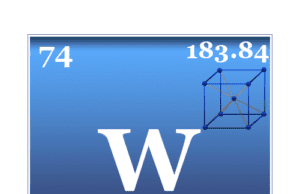
Tungsten
Tungsten Metal
Tungsten is a chemical element or strong, silvery transition metal of Group 6 (VIB) of the periodic table with the symbol W and atomic...
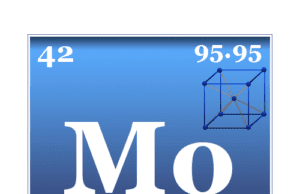
Molybdenum
Molybdenum Metal
Molybdenum is a chemical element or strong and silvery transition metal of Group 6 (VIB) of the periodic table with the symbol Mo and...
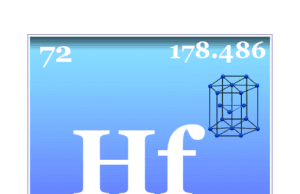
Hafnium
Hafnium Element
Hafnium is a chemical element or silvery-white transition metal of Group 4 (IVB) of the periodic table with the symbol Hf and atomic number...