Molybdenum Metal
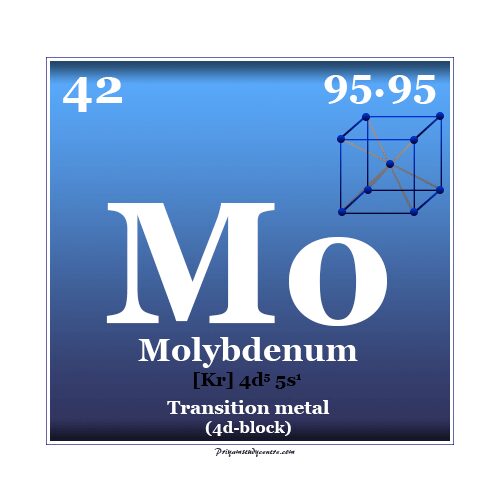
Molybdenum is a chemical element or strong and silvery transition metal of Group 6 (VIB) of the periodic table with the symbol Mo and atomic number 42. The metal molybdenum increases the mechanical strength, electrical conductance, and corrosion resistance of steel. Therefore, it is used mainly for making stainless steel and alloy steel for high-speed tools. The name molybdenum is given from the Greek word molybdos meaning lead. It is the element in the second transition or 2d series in which all the d-electrons participate in metallic bonding and is placed with chromium and tungsten on the periodic table. The molybdenum metal form is typically a metallic body-centered cubic crystal lattice. It is hard and relatively unreactive due to the protective surface film of oxide.
Who Discovered Molybdenum?
The soft black molybdenite (MoS2) is the principal ore of molybdenum used for writing in the old times. It was similar to that of lead ore (galena or PbS) and graphite.
The mineral was first studied in 1754 by Swedish mineralogist A. Cronstedt. Following him, in 1778 Swedish chemist Carl Wilhelm Scheele observed that it was neither galena nor graphite. He prepared the oxide of molybdenum metal.
The pure metal was prepared by Berzelius in 1817 during the reduction of the oxide with hydrogen. The name of the metal is given from the Greek word molybdos meaning lead.
Properties
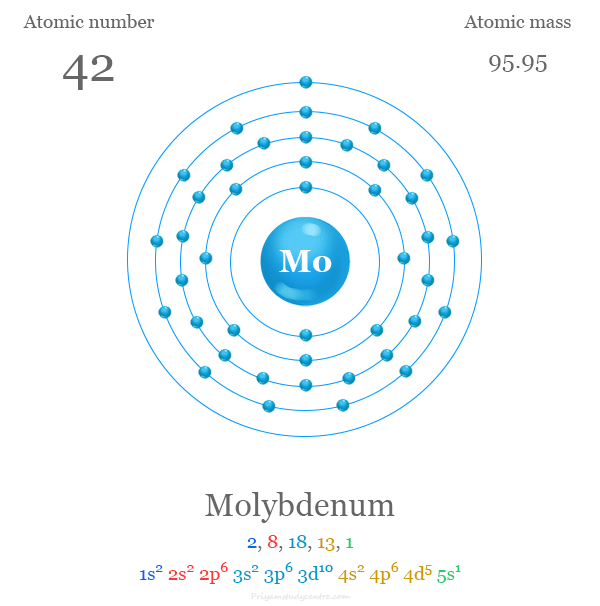
The atomic number of Mo is 42 and the valence shell electronic configuration [Kr] 4d5 5s1. The most common oxidation number or state of molybdenum metal is +6 (VI). The oxidation states V and IV are also stable. Strong reducing properties of molybdenum metal not met before III and II states.
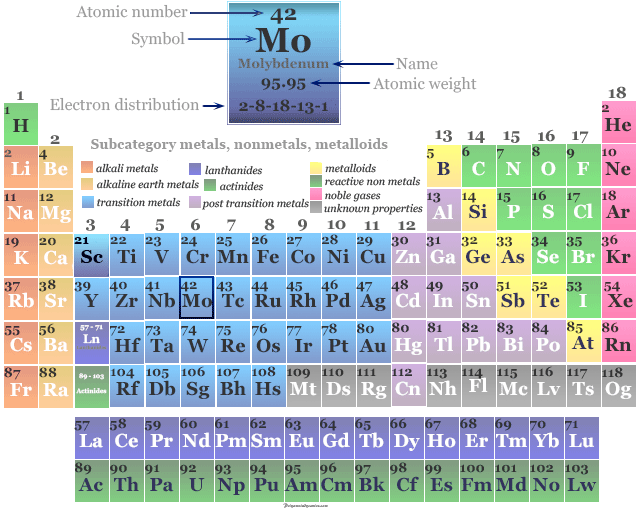
| Properties of Molybdenum |
|||
| Atomic number | 42 | ||
| Electron per shell | 2, 8, 18, 13, 1 | ||
| Electronic configuration | [Kr] 4d5 5s1 | ||
| Block | d-block | ||
| Period | period 5 | ||
| Group | group 6 | ||
| Atomic weight | 95.94 | ||
| Melting point | 2896 K (2623 °C, 4753 °F) | ||
| Boiling point | 4912 K (4639 °C, 8382 °F) | ||
| Crystal structure | |||
| Density | 10.28 g/cm3 | ||
| Molar heat capacity | 24.06 J mol−1K−1 | ||
| Electrical resistivity | 53.40 nΩ·m | ||
| Atomic radius | 139 pm | ||
| Covalent radius | 154±5 pm | ||
| Chemical properties | |||
| Common oxidation number | +6 | ||
| Electronegativity | Pauling scale: 2.16 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 684.3 | 1560 | 2618 | |
Source of Molybdenum
The metal molybdenum and tungsten have very poor amounts in the earth’s atmosphere. It is the 54th most abundant element in the Earth’s crust with an occurrence of 1.5 parts per million.
The most important ore of Mo is molybdenite (MoS2). It is found mostly in Colorado (USA), Canada and Chile. Other minerals of the Mo metal are wulfenite (PbMoO4) and molybdite (MoO3).
Foods that Contain Molybdenum
It is also found in food grains, vegetables, and animal products. The richest sources of molybdenum are legumes, whole grains, nuts, milk, and beef liver. It is the only element or metal in the second transition series that has natural biological functions.
Milk, cheese, and eggs are the main sources of molybdenum metal for children teenage boys, and girls. Our daily drinking water also contains some amount of Mo-metal.
Isotopes of Molybdenum
Molybdenum has 35 known isotopes with an atomic mass of 83 to 113. It has seven naturally occurring isotopes with atomic masses 92, 94, 95, 96, 97, 98, and 100.
The radioactive isotopes of the metal are obtained by different types of nuclear reactions. 93Mo is the most stable radioisotope of the metal with a half-life of 4,000 years.
Production Process
- Molybdenite was concentrated by foam floatation and roasted to MoO3.
- First, it is reduced to MoO2 by heating with hydrogen at 500 °C.
- In the second step, MoO2 is reduced by hydrogen at 1100 °C to get the metal.
- Pure molybdenum metal is obtained by hydrogen reduction of ammonium molybdate. Carbon reduction of MoO3 gives mostly carbides.
Ferromolybdenum is prepared by reducing molybdenite with iron and coke in an electric furnace. It is also prepared by reducing a mixture of Mo and Fe oxides with aluminum (thermite reaction).
The metal has a very high melting point and is obtained initially in the form of molybdenum powder. It is converted to massive states by compression under hydrogen at high temperatures.
Chemical Compounds
The chemistry of transition metals molybdenum and tungsten are very similar. The metals are slightly attacked by weak acids and bases. Both of them are dissolved in a nitric acid – hydrogen fluoride mixture and in fused alkalies. The oxide compounds are acidic but the salts derived from acids are complex polyanions.
The common and stable oxidation states of these metals are +6 (VI). The oxidation states V and IV are also stable. Strong reducing properties of molybdenum are not observed before the III and II states. In the VI state, the metal forms oxides, oxoacids, hexafluorides, and hexachlorides.
Molybdenum Oxide
Molybdenum forms hexavalent oxide like MoO3. It is precipitated from alkali molybdates by the addition of acids. The precipitated H2MoO4 may be ignited to form oxides. The oxide may also be prepared by ignition of metal in air or oxygen.
All the oxides are acidic and dissolved in alkali to give molybdates. The oxide CrO3 is highly oxidizing in nature. The oxides like MoO2 and WO2 have no oxidizing properties.
The lower oxide MoO2 was obtained by the reduction of trioxide with hydrogen. The acidic nature of the oxide decreases with the decreasing oxidation state.
Molybdenum Halides
With the high oxidation number, molybdenum halides are expectedly covalent in nature. Therefore, MoF6 is a low boiling, nonelectrolyte, diamagnetic, and extremely dissolved to hydrolysis. MoCl5 is monomeric in the vapor state but a bridged dimer in the crystalline state. The lower halides are very complicated structures.
The higher halides are obtained by the direct halogenation of the metal. However, the lower halides are obtained by the reduction of higher halides with metal or hydrogen under optimized temperature and pressure.
Molybdenum Complexes
It forms monomeric MO4−2 anions in an alkaline medium. On acidification, the anions polymerize to give polyacids. These give rise to a complex like dioxomolybdenum(VI).
The pentavalent complexes are mostly of the oxo-molybdenum or di-μ-oxo molybdenum type. Some common examples of Mo(IV) complexes are [MoCl5O]−2, [Mo(acac)2ClO]−2, etc. Tetravalent complexes are both oxo and nonoxo types.
In a lower oxidation state, molybdenum generally forms Mo6X12 cluster compounds (where X = chlorine, bromine, and iodine). In this type of compound six Mo atoms form cluster-type compounds like (MoX8)+4X4−. The cyano complex may also be prepared by reducing the Mo(IV) complex. It has a pentagonal bipyramid structure.
Uses of Molybdenum
- The main consumption of molybdenum is used generally in the manufacturing of stainless steel and other types of steel used in high-speed tools. The oxide MoO3 and ferromolybdenum are directly used for this purpose.
- Molybdenum increases the mechanical strength, electrical conductance, and corrosion resistance of steel. Therefore, Ni-Mo steel is used for making gun barrels and propeller shafts. Cr-Mo steel is used for making aircraft.
- Pure molybdenum metal is also used to treat crude petroleum with hydrogen gas. The metal and hydrogen convert sulfur-containing organic compounds to H2S.
- Molybdenum metal is used as a chemical catalyst.
- The compound MoS2 has a layer of crystal lattice and is used for making high-temperature lubricants.
Biological Function
It is the only element or metal in the second transition series that has natural biological functions. More than thirty microorganisms, plants, and animals are known to contain the metal.
Nitrogen fixation enzyme nitrogenases contain a characteristic polymetallic cluster with the Fe-Mo cofactor. Other enzymes function like oxidase, reductase, and dehydrogenase have also variant types of molybdenum metal cofactors.