Hydrocarbon
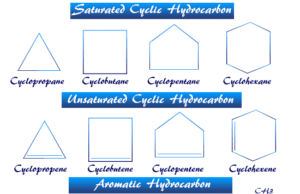
Types of Hydrocarbons with Examples
Hydrocarbon (aliphatic, aromatic, or polynuclear hydrocarbon) is a type of organic compound that processes hydrogen and carbon in the entire molecular...
Chemical Bonding
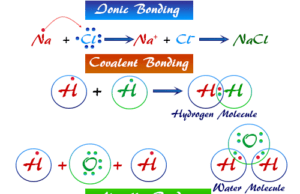
Chemical Bonding in Chemistry
Chemical bonding or chemical bond in learning chemistry is the different types of forces that bind together two common atoms or...
Environmental chemistry
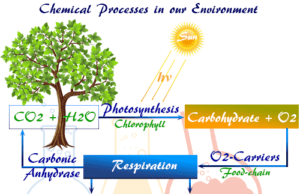
Chemistry and Environmental Science
Environmental chemistry is the branch of science where we study abiotic, biotic, energy components, and chemical elements of the ecosystem of...
Radioactive Isotopes
What are Radioactive Isotopes?
Radioactive isotopes or radioisotopes of a radioactive element are atoms of the same element with different mass numbers. Isotopes of a...
Ionization Energy
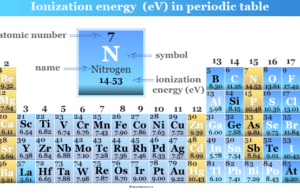
Ionization Energy in Periodic Table
Ionization energy or ionization potential in chemistry is the minimum amount of energy required to remove the outer electron of...
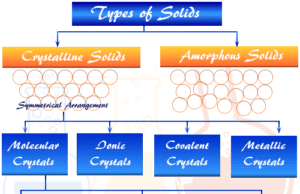
Crystalline Solids
Crystalline Solids and Amorphous Solids
Crystalline solids and amorphous solids are the two types of solid materials formed by atoms, ions, or molecules. Molecular, ionic, covalent,...
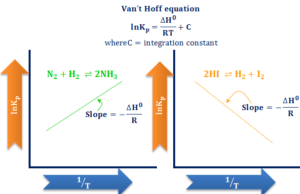
Van’t Hoff Equation
Van't Hoff Equation-Effect of Temperature on Equilibrium Constant
Van't Hoff equation connecting chemical equilibrium constant (Keq) and temperature by thermodynamics relation of Gibbs-Helmholtz free energy...
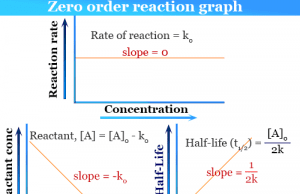
Zero Order Reaction
Zero Order Reaction Kinetics
Zero order reaction kinetics in chemistry define the rate of chemical reaction in terms of reactant and product per unit time....
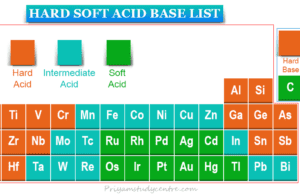
Hard Soft Acid Base
Hard Soft Acid Base List
Hard soft acid base theory or HSAB principle was proposed by Ralph Pearson in 1963. HSAB principle proposed that the...
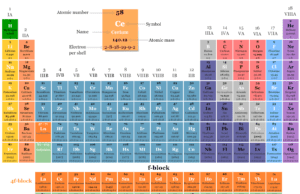
f block Elements
f Block Elements on Periodic Table
f block elements appear in two chemical series like 4f block names as lanthanides or rare earth elements and...