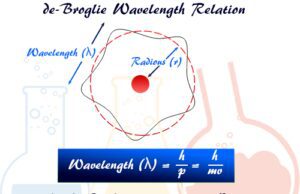
de Broglie Relation
de Broglie Wavelength Formula
de Broglie wavelength formula proposed by French physicist Louis de Broglie in 1924 derived the relation between Einstein's mass-energy equation and...
Oxidation Number
Oxidation Number of Periodic Table Elements
Oxidation number or state of periodic table elements in a chemical compound or molecule is the formal charge (positive...
Electron Affinity
Electron Affinity in Periodic Table
Electron affinity (EA) or electron gain enthalpy or simply affinity in the periodic table defines the amount of energy released...
Slater’s Rule
Slater's Rule for Calculating Shielding Constant
Slater's rule is an empirical set of rules in chemistry for calculating the shielding constant or screening constant (σ)...
Ideal Gas Law
Ideal Gas Law Equation
Ideal gas law or perfect gas law represents the mixed relationship between pressure, volume, and temperature of hypothetical ideal gases for...
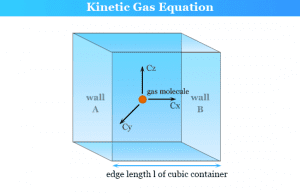
Kinetic Theory of Gases
Kinetic Molecular Theory of Gases
Kinetic molecular theory of gases and the kinetic gas equation was first developed by Bernoulli in 1738 to derive the molecular...
Dipole Moment
Bond Moment and Dipole Moment
Dipole moment formula in chemistry is used to find out or calculate the net bond moment and polar character or...
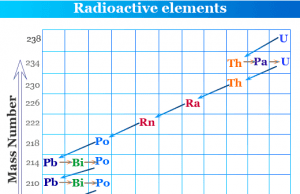
Radioactivity
Measurement of Radioactivity
Radioactivity is the phenomenon of emission of radiations as a result of the spontaneous decay of alpha, beta, and gamma particles or...
Balancing Chemical Equations
Balancing Chemical Equations in Chemistry
Balancing chemical equations by ion-electron formula and change of oxidation number are used generally to balance the oxidation reduction process...
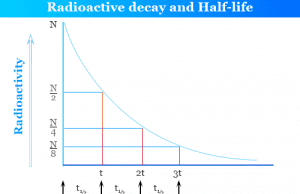
Radioactive Decay
Radioactive Decay and Half Life
Radioactive decay is the spontaneous disintegration or emission of atomic particles like alpha, beta, gamma from the nuclei of radioactive...