Lewis Acid and Base Definition
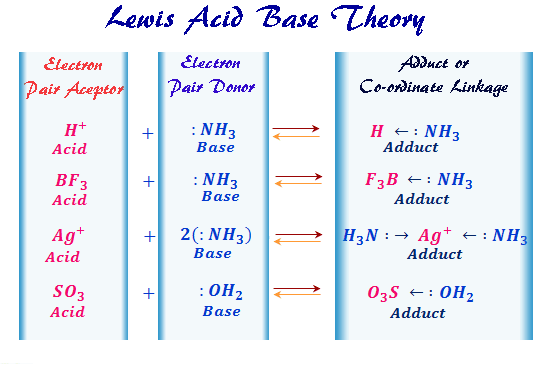
Lewis acid base concept or theory explains the acids and bases properties in terms of electronic structure with the formation of the coordinate covalent chemical bond in chemistry. In learning chemistry, the Lewis acids and bases concept does not restrict to the concentration of hydrogen ions or the pH scale of the solution. It is based on the electronic configuration or structure of atoms, ions, or molecular species. According to American chemist Gilbert Newton Lewis, an acid is any species (molecule, radical, or ion) that can accept an electron pair to form a coordinated covalent bond. Similarly, a base is any species that can donate an electron pair to the coordinate covalent bond. In an acid base chemical equilibrium reaction, a base leads to form a coordinate link to the acid. An acid must have a vacant orbital in which the electron pair donated by a base can be accommodated. A Lewis base is a substance that has at least one unshared (lone) pair of electrons.
Lewis Acid Base Theory
A (Lewis acid) + :B (Lewis base) → A : B (Adduct)
According to Lewis theory, the neutralization reaction is simply for the formation of the co-ordinate bond between an acid and a base. The neutralization product is called acid-base complex or adduct. It is either non-ionizable or may undergo ionization depending on its stability.
Lewis Acids and Bases Examples
| Chemical reaction | Lewis acid | Lewis base | Adduct or addition complex |
| Ammunition | Ag+ | 2 :NH3 | [Ag(NH3)]+ |
| Cu+2 | 4 :NH3 | [Cu(NH3)4]+2 | |
| Hydration | Co+3 | 6 :OH2 | [Co(H2O)6]+3 |
| Alcoholation | Li+ | :OHCH3 | [Li(CH3OH)]+ |
In the Lewis acid-base neutralization reaction, an acid acts as an electron acceptor, and the base is considered an electron-pair donor. In the above reactions, we provide some examples of Lewis acids and bases with acid base complexes. The stability of such complexes can be explained by the hard soft acid base theory.
Arrhenius Acid Base Theory
Arrhenius defined, that acids when dissolved in water dissociate into hydrogen ions and anions. Therefore, hydrogen ion (H+) is released from nitric acid, and sulfuric acid acts as an acid.
The hydrogen ion has an affinity to receive an electron pair from the base. Thus all the Arrhenius acids are also acids under the Lewis definition.
Bronsted Lowry Acid Base
According to the protonic definition by Bronsted-Lowry, a base is any molecule or ion, which accepts a proton. According to Lewis definition, an acid is a molecule that has an atom capable of sharing a pair of electrons with a base.
During the neutralization reaction, a coordinate covalent bond is formed between the donor atom of a base and the proton or hydrogen ion of an acid. Hence all Bronsted-Lowry bases are bases in the Lewis sense also.
Question: What is the conjugate acid base pair of bisulfate?
Answer: The molecular formula of bisulfate ion is HSO3−. It may lose one hydrogen ion to give the conjugate base SO3−2. Again it may use hydrogen ions to give the conjugate acid, H2SO3−.
Solvent System Concept
The solvent system concept recognizes that ammonium chloride should behave as an acid in liquid ammonia, alcohol, or acetic acid solution. However, an ammoniated hydrogen ion can accept a lone pair of an electron from the base. Therefore, an ammonium ion in a liquid ammonia solution is an acid according to Lewis’s sense.
According to the solvent system concept, an amide ion is a base in liquid ammonia. The amide ion is capable of donating a lone pair to an acid. Hence amide ion is a base according to Lewis theory.
Question: What type of chemical bonding is formed, when sulfur trioxide reacts with water?
Answer: Sulfur trioxide (SO3) like boron trifluoride (BF3) has less than an octet and will be termed an acid according to Lewis concept. In water oxygen atoms contain lone pairs to donate the Lewis acid sulfur trioxide.
Classification of Lewis Acid and Base
A wide variety of chemical compounds are recognized as an acid or a base according to Lewis theory. Lewis theory can be classified acids and bases into the following categories,
- Incomplete octet of the central atom
- Elements having vacant d orbital
- Cations of an atom
Incomplete Octet of the Central Atom
Electron deficient molecules such as alkyl and halides of beryllium, boron, and aluminum are examples of these classes of acids. Some reactions of these types of Lewis acids and bases are,
F3B + O(C2H2)2 ⇄ F3B ← :O(C2H5)2
Cl3Al + NC5H5 ⇄ Cl3Al ← :NC5H5
Me3B + N2H4 ⇄ Me3B ← :N2H4
Question: What happens when BF3 reacts with NH3?
Answer: In ammonia, the central nitrogen atom has a lone pair of electrons. This lone pair coordinates with the empty orbital of an atom. Therefore, ammonia is a base according to Lewis.
The compounds with less than an octet for the central atom are Lewis acids. In BF3, the central boron atom contains six electrons. Therefore, it behaves as an acid.
Elements having Vacant d Orbital
The central atom of the halides such as SiX4, GeX4, TiCl4, SnX4, PX3, PF5, SF4, SeF4, and TeCl4 has vacant d-subshell. These chemical substances can accept an electron charge pair from the Lewis base to accommodate in their vacant d-subshell.
The central atom of these compounds forms adducts with halide ions, oxygen, nitrogen, and sulfur-containing organic hydrocarbon. These substances are thus Lewis acids.
These halides are vigorously hydrolyzed by water to form an oxyacid or oxide of the central atom. The hydrolytic reaction takes place through the formation of an unstable Lewis acid base adduct (intermediate) with water.
Problem: Why PCl3 can act both as Lewis acid and Lewis base?
Solution: PCl3 has a lone pair of electrons in phosphorus. This lone pair may coordinate with a metal ion thus the compound has the properties of Lewis base.
Ni + 4 PCl3 ⇄ [Ni(PCl3)4]0
Again the quantum shell of phosphorus has provision for d sub-orbital which can receive back donated electrons from electron reach low oxidizing agents or metal ions. In this latter case, the tri-covalent phosphorus compound serves as a Lewis acid.
Cation of an Atom
Theoretically, all simple cations (silver, copper, cobalt, and lithium-ion) are potential Lewis acids. Therefore, the reactions of these cations by Lewis acids base theory given below,
Ag+ + 2 :NH3 ⇄ [H3N: → Ag ← :NH3]+
Cu+2 + 2 :NH3 ⇄ [Cu(NH3)4]+2
Co+3 + 6 :OH2 ⇄ [Co(OH2)6]+3
Li+ + : OHCH3 ⇄ [Li ← :OHCH3]
Lewis Acids and Bases Strength
The Lewis acid strength or coordinating ability of the simple cations is increased with an increase in the positive charge carried by the cation.
- With an increase in the charge on the nucleus or increasing the atomic number of the metals in any period of the periodic table, acid strength is increasing.
- It decreases by decreasing the ionic radius and the number of shielding electron shells.
- Hydrogen bonding also affects acid-base strength.
The Lewis acid strength of simple cations increases when we move from left to right in a period or from bottom to top in a group of the periodic table.
- Fe+2 < Fe+3 (positive charge increases)
- K+ < Na+ (on moving from bottom to top in a group)
- Li+ < Be+2 (on moving from left to right in a period)
Example of Lewis Acid
Molecules having multiple electric polarization between atoms of dissimilar electronegativity like carbon dioxide, sulfur dioxide, sulfur trioxide, borax, hydrogen peroxide, and boric acid are examples of Lewis acids.
In these compounds, the oxygen atom is more electronegative than the central atom. As a result, π-electron density is displaced away from the central atom.
These are electron deficient chemical compounds able to accept the electron pair from Lewis bases. They form a dative bond with very low bond energy.
Acidic Nature of Oxides
Acidic oxides react with water to give oxoacids in redox reactions. The higher the oxidation number and higher the electronegativity the greater the central chemical element will force to react with water to give the oxoacid.
Acidic nature of oxides: Na2O < MgO < As2O3 < N2O5 and oxidation number of oxide-forming element = +1, +2, +3, +5
Utility of Lewis Acid Base Concept
- Lewis acids bases concept includes those chemical reactions in which no hydrogen atom or ion is involved.
- The concept is more general than the Bronsted-Lowry concept or protonic concept.
- The acid base properties are not dependent on the presence of one particular element or on the presence or absence of a solvent.
- It explains the long accepted basic properties of metallic crystalline solid oxides and the acidic properties of non-metallic oxides.
- The Lewis concept or theory explains many acid base reactions in the gas phase in high temperatures and non-solvent reactions as the neutralization process.