Titanium Metal
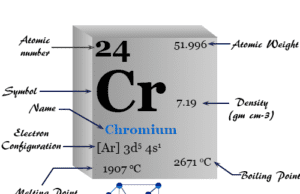
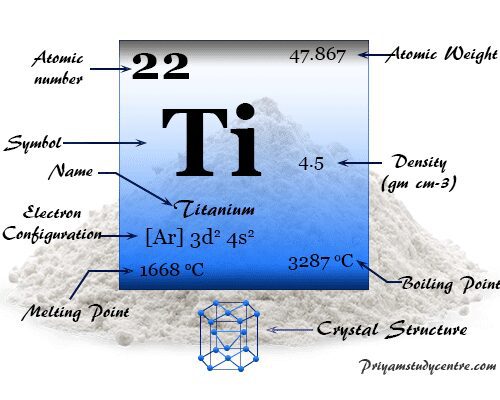
Titanium is a chemical element or light silvery metal of Group 4 (IVB) in the periodic table with the symbol Ti and atomic number 22. It has a high melting point, good tensile strength, and properties of thermal and electrical conductance. It forms a hexagonal close-packed crystal lattice like most of the other transition metals. The valence shell electronic configuration of metal is 3d2 4s2. Therefore, the highest and most stable oxidation number or state of titanium is +4. Ti is a very useful metal that uses widely in the chemical industry and for making surgical types of equipment. The metallic titanium is generally alloyed with manganese, chromium, iron, molybdenum, aluminum, vanadium, and tin. Titanium alloy is mostly used in high-speed aircraft and missile industries due to its lightness and mechanical strength.
Who Discovered Titanium?
In 1791, the English chemist and mineralogist William Gregor attempted to discover a new metal from titaniferous iron ore, ilmenite (FeTiO3). But he actually isolated an impure oxide.
In 1794, German chemist Martin Heinrich Klaproth prepared the same oxide from mineral rutile. The name of the element, titanium comes from the Greek word Titans.
Titanium Properties
Good qualities, corrosion resistance Ti has valence shell electron configuration [Ar] 3d2 4s2. Therefore, the chemistry of titanium dominated at the +4 oxidation state.
| Titanium | |||
| Chemical symbol | Ti | ||
| Discovery | William Gregor in 1791 | ||
| Name derived | Word Titans, (sons of Earth goddess in Greek mythology) | ||
| Common isotope | 48Ti | ||
| CAS number | 7440-32-6 | ||
| Periodic Properties | |||
| Atomic number | 22 | ||
| Atomic weight | 47.867 | ||
| Electron per shell | 2, 8, 10, 2 | ||
| Electronic configuration | [Ar] 3d2 4s2 | ||
| Group | 4 | ||
| Period | 4 | ||
| Block | d-block | ||
| Physical Properties | |||
| State at 20 °C | Solid | ||
| Malting point | 1668 °C | ||
| Boiling point | 3287 °C | ||
| Density | 4.50 gm/cm3 | ||
| Molar heat capacity | 25.06 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Electrical resistivity | 420 nΩ⋅m at 20 °C | ||
| Chemical Properties | |||
| Atomic radius (non-bonded) | 2.11 Å | ||
| Covalent radius | 1.48 Å | ||
| Oxidation states | 4, 3 | ||
| Electronegativity | 1.54 (Pauling scale) | ||
| Electron affinity | 7.622 kJ mol−1 | ||
| Ionization energy | 1st | 2nd | 3rd |
| 658.81 | 1309.84 | 2652.55 | |
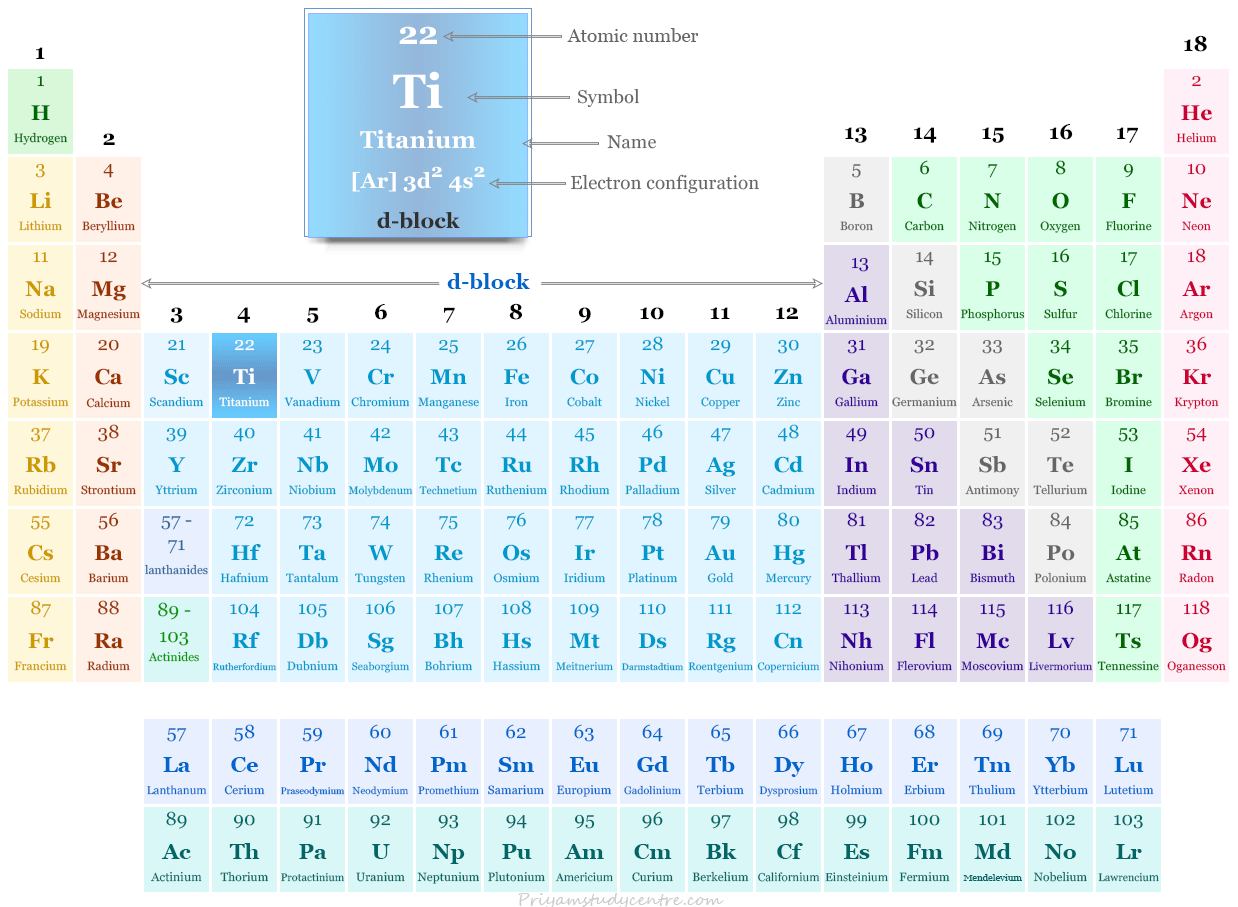
Titanium in the Periodic Table
The transition metal Ti is placed in group 4 and period 4 of the periodic table. It is a d-block element that lies between scandium and vanadium.
Where is Titanium Found?
Titanium is the ninth most abundant among all elements and second, among the transition metals. It constitutes nearly 0.63 percent of the earth’s crust. The combined form of metal is found mostly in igneous rocks, sand, clay, and soils, in living organisms (plant and animal), and in natural bodies of water.
The metal was isolated by metallurgist Berzelius in 1825 and the pure form by M. Hunter in 1910 by reducing titanium chloride (TiCl4) with an airtight steel cylinder.
Ilmenite (FeTiO3) and Rutile (TiO2) are two commercial ores of titanium. It mostly occurs in Western Australia, Canada, China, South India, Mozambique, Malaysia, New Zealand, Norway, Sierra Leone, South Africa, and Ukraine.
Titanium Isotopes
Natural titanium consists of five stable isotopes such as 46Ti (8.0 percent), 47Ti (7.3 percent), 48Ti (73.8 percent), 49Ti (5.5 percent), and 50Ti (5.4 percent).
Production Process
The preparation of pure titanium is very difficult due to its reactivity at high temperatures. The extraction of metal by carbon reduction causes a number of difficulties. It is highly reactive at high temperatures and readily forms carbide, nitride, and oxide by the reaction with carbon, oxygen, and nitrogen respectively.
The extraction of the metal is carried out by heating ilmenite or rutile with carbon and chlorine at 900 °C.
Titanium tetrachloride (TiCl4), boiling point of 137 °C, is separated from FeCl3 by fractional distillation.
When TiCl4 is reduced by molten magnesium in an argon atmosphere it produces spongy titanium metal.
TiCl4 + 2 Mg → Ti + 2 MgCl2
Magnesium chloride and excess magnesium are removed by washing water and dilute hydrochloric acid.
Pure titanium is mostly obtained by heating the metal with iodine in an evacuated glass tube fitted with a tungsten filament at the center of the tube.
Facts About Titanium
- Titanium is a high melting, good tensile strength, and corrosion resistance transition metal present in Group 4 of the periodic table.
- The metal is unreactive at ordinary temperature but in a finely divided state, it catches fire in the air (pyrophoric).
- On heating, it combines with nonmetals like oxygen, nitrogen, boron, carbon, silicon, and hydrogen.
- The nitride (TiN), carbides (TiC), and borides (TiB, TiB2) are very hard, a good conductor of electricity, and chemically very inert compounds.
- The metal decomposes by steam at 100 °C but is not attacked by dilute mineral acids like sulfuric acid, hydrochloric acid, or nitric acid at ordinary temperature.
- It does not attack by hot aqueous alkali but fused alkali attacks it to give titanates.
Chemical Compounds
In learning chemistry, the valence shell electron configuration of titanium is 3d2 4s2. Therefore, the highest and most stable oxidation state of Ti is +4. The compounds of a lower oxidation state (0, II, III) readily oxidized to form Ti (IV).
The high ionization energy is required to form the Ti+4 ion. It suggests that the chemical compounds of titanium in the +4 state are formed by covalent bonding.
Titanium Oxide
Titanium oxide (TiO2) has three crystalline solid forms, rutile, anatase, and brookite. However, rutile is the most common naturally occurring form which uses in chemical industries as a pigment. In all the structures, Ti is coordinated to six oxygen atoms.
Ti2O3, violet color oxide of Ti (III) has a structural type similar to that of α-Al2O3.
TiO may be made by heating TiO2 with metallic titanium. It is from a cubic crystalline structure like sodium chloride but usually non-stoichiometric with one-sixth vacant sites for both ions. It is used as a metallic conductor.
Titanium Disulfide
The disulfide (TiS2) is the most important sulfide compound of titanium formed with sulfur atoms. It consists of a layer structure. It is used as an electrode for the development of lithium batteries.
Halides
All four halides TiF4, TiCl4, TiBr4, and TiI4 are known. TiF4 may be obtained during the reaction between Ti and fluorine at 200 °C. Other tetra-halides may be prepared by heating TiO2 with carbon and halogen.
In the +3 oxidation state, it also forms all four halide molecules. They are insoluble in water and stable in air. They are disproportionate to form Ti (IV) halides.
Organometallic Compounds
Organometallic compounds of titanium were developed in 1960 by the discovery of the Ziegler Natta chemical catalyst. A large number of organometallic compounds are also obtained by chemical bonding like sigma and pi-bond.
The most common organotitanium complex is titanocene dichloride [(C5H5)2TiCl2]. Tebbe’s reagent and Petasis reagent are the related compounds of titanocene dichloride.
Uses of Titanium
- Due to its high efficiency and low biological toxicity, the titanium (IV) complex is the first non-platinum compound tested for the treatment of cancer in medicine.
- It is an impotent alloying material due to its low density, high tensile strength, and excellent corrosion resistance. The addition of 0.1 percent of titanium to steel increases the mechanical strength and corrosion resistance of the alloy.
- Titanium alloys are also used in many chemical and industrial fields like storing alkaline solutions, chlorine compounds, and other aggressive chemicals
- Titanium alloys are also used for making rails, railway wheels, and excels.
- Its alloys with manganese, chromium, iron, molybdenum, aluminum, vanadium, and tin. These alloys are used in the aeronautical and missile industries due to their lightness and mechanical strength.
- Ferrotitanium is prepared by smelting ilmenite or rutile with iron and coke in an electric furnace. Therefore, it is used as a scavenger in the steel industry to remove oxygen and nitrogen from steel.
- Titanium oxide (TiO2) is extensively used as white pigment due to its excellent covering power, which is prepared along the same route of metal extraction.