Selenium Element
Selenium is a group-16 chemical element on the periodic table with the symbol Se and atomic number 34. It is placed with group 16 elements such as oxygen, sulfur, tellurium, and polonium. Selenium uses mainly for making decolorized glass, photoelectric cells, and semiconductor devices. It is an essential component of various enzymes and proteins. Selenium formed a selenide mineral with sulfides of copper, silver, mercury, and nickel. The element selenium has properties of nonmetal. It is recovered from the anode mud obtained in the electrolytic refining of copper. Selenium is a trace mineral found in several foods and necessary for cellular function in many animals. Selenium is a trace mineral found in several foods and necessary for cellular function in many animals. It is an essential component of various enzymes and proteins.
Selenium formed a selenide mineral with sulfides of copper, silver, mercury, and nickel. The element selenium has properties of nonmetal. It is recovered from the anode mud obtained in the electrolytic refining of copper.
The name selenium was derived from the latter ‘selene’ the Greek name for the Moon. It was discovered by Jöns Jacob Berzelius and Johan Gottlieb Gahn in 1817.
It forms five, six, seven, and eight-membered rings but they are readily converted to more stable chains. Infinite long chains are present in some solid nonmetals.
It has common rhombic and monoclinic forms with Se8 puckered rings. Both forms are unstable and slowly change to gray polymeric forms with infinite chains.
Sources
It is found as selenide minerals along with sulfides of copper, silver, mercury, nickel, etc. Commercially, most of the selenium is obtained as a by-product of copper extraction and purification.
Natural foods are also other sources of selenium which is required in many biological or cellular functions. Plants can obtain it from soil and animals gain it from plant sources.
Proteinous foods such as Brazil nuts, finfish, shellfish, beef, turkey, chicken, fortified cereals, whole-wheat bread, beans, and lentils are the main sources of selenium that are required for many biological processes.
Americans can fulfill their daily required Se element from bread, cereals, poultry, red meat, and eggs.
Production Process
Selenium is produced mostly from the anode mud obtained from the electrolytic refining of copper. The dried mud contains about 3 to 28 percent Se.
- The dried mud can be roasted with sodium bicarbonate in the air at 650 °C to form sodium selenite.
- Sodium selenite obtained by this process is leached with water.
- The product can be neutralized with sulfuric acid to form selenous acid solutions.
- Se is produced from selenous acid solutions by reduction with sulfur dioxide.
H2SeO3 + 2SO2 + H2O → 2H2SO4 + Se
Properties of Selenium
It has properties to form several types of allotropes. Gray Se element is the only important allotrope of the element. It has infinite spiral chains of atoms.
The viscosity of molten selenium decreases rapidly because of long-chain split into smaller ones by increasing temperature. A highly polymeric form is obtained by pouring molten Se into cold water.
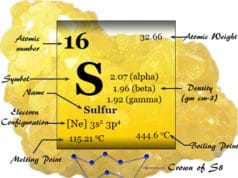
Many chemical properties of selenium are given below table,
| Properties of selenium | |
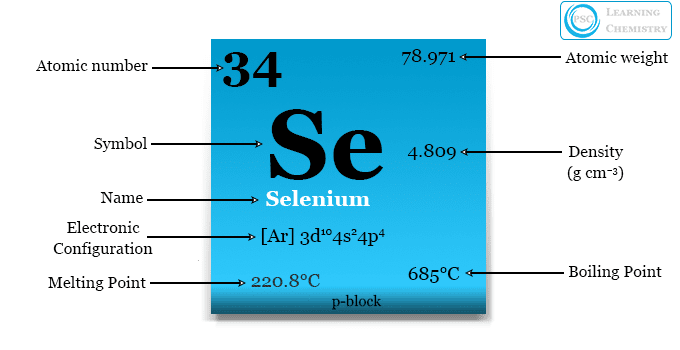
| Symbol | Se |
| Atomic number | 34 |
| Discovered by | Jöns Jacob Berzelius |
| Origin of the name | derived from ‘selene’, the Greek name for the Moon |
| Electron per shell | 2, 8, 18, 6 |
| Electron configuration | [Ar] 3d10 4s2 4p4 |
| Group | group 16 (chalcogens) |
| Period | period 4 |
| Block | p-block |
| State at 20°C | Solid |
| Density (g cm-3) | 4.809 |
| Melting point | 220.8°C |
| Boiling point | 685°C |
| Critical temperature | 1766K |
| Oxidation states | −2, −1, +1, +2, +3, +4, +5, +6 |
| Ionization energy | 1st: 941.0 kJ/mol |
| 2nd: 2045 kJ/mol | |
| 3rd: 2973.7 kJ/mol | |
| Electronegativity | 2.55 (Pauling scale) |
The ionization energy of group-16 elements such as O, S, Se, Te, and Po is very high. It can be decreased down the group. Therefore, the metallic character of group-16 elements increases with the increasing atomic number.
Hence, sulfur is an insulator, selenium, and tellurium are semiconductors, and polonium is a metal.
Selenium in the Periodic Table
The electronic configuration of nonmetal Se is [Ar] 3d10 4s2 4p4. Therefore, selenium is found in Group 16 and Period 4 in the periodic table.
It is a p-block element present below the sulfur and above tellurium.
Selenium Compounds
The elements of group-16 or chalcogens have just two electrons short of the next noble gas configurations. Therefore, the −2 oxidation state is the most common oxidation state of Se.
Selenium compounds also exist in +2, +4, and +6 oxidation states. The properties and production of the most common selenium compounds are discussed below,
Hydrogen Selenide
Hydrogen selenide can be obtained by the hydrolysis of aluminum selenide in water or by diluting non-oxidizing acid.
Al2Se3 + 3 H2O → 3 H2Se + Al2O3
The hydride can also be produced by heating finely powdered selenium with long-chain hydrocarbon at 300 to 400 °C. It is used in synthetic organic chemistry for the dehydrogenation of hydrocarbons. H2Se is more soluble in water than H2S.
Selenium Dioxide
Selenium dioxide forms colurless sublime crystals which melt under pressure at 340 °C. It is highly soluble in water. The evaporation of water solution forms colurless hexagonal crystals of selenous acid.
It is less stable than SO2 and easily reduced to Se by SO2. SeO2 is used largely in organic chemistry as an oxidizing agent.
Selenium Trioxide
The trioxide (SeO3) is formed along with dioxide on passing an electric discharge through a mixture of selenium vapour and oxygen under low pressure. It is the anhydride of H2SeO4. In solid-state, it contains a cyclic Se-O-Se trimer.
Selenous Acid
Selenous acid crystallized from an aqueous solution of SeO2. It is also formed by oxidizing selenium powdered with dilute HNO3.
SeO2 + H2O → H2SeO3
3 Se + 4 HNO3 + H2O → 3 H2SeO3 + 4 NO
Selenous acid gives two series of salts such as trioxoselenates (SeO3−2) and hydrogen trioxoselenates (HSeO3−). It is oxidized by ozone to give selenates.
On the other hand, it can be oxidized by H2S, SO2, and HI to produce powdered Se.
H2SeO3 + 2 H2S → Se + 2 S + 3 H2O
H2SeO3 + 2 SO2 + H2O → Se + 2 H2SO4
2 HI + H2SeO3 → Se + 2 I2 + 3 H2O
Selenic Acid
- Selenic acid is obtained from the oxidation of H2SeO3 by 30 percent hydrogen peroxide.
H2SeO3 + H2O2 → H2SeO4 + H2O - It is also produced from selenous acid by oxidation with KMnO4.
- Selenic acid can be produced from Se by oxidation with chlorine water.
Se + 3 Cl2 + 4 H2O → H2SeO4 + 6 HCl
Selenic acid forms white deliquescent crystals which readily lose water on heating. The aqueous solution of selenic acid is a strong acid that dissolves metals like copper, silver, gold, and palladium. It liberated hydrogen with zinc.
Halides of Selenium
The important halides are SeX4 (where X = F, Cl, Br) and SeF6.
- SeF4 is a colorless fuming liquid obtained by regulated fluorination of Se at 0°C. It is also obtained by the reaction of SF4 with SeO2 above 100 °C.
- SeF6 is obtained by reducing Se with fluorine.
Selenium Uses
- The chief commercial uses of selenium are making and decolorizing glasses. At higher concentrations, it gives a pink shade to glass.
- Selenium compounds such as cadmium sulfoselenide are used to make beautiful ruby-red glasses.
- Xerography or photocopying is based on the photoconductive properties of selenium.
- It is used to make rectifiers in semiconductor devices and photoelectric cells
- Ferroselenium is used for alloying stainless steel. It is also used with bismuth in brasses to replace more toxic elements like lead.
- The lithium–selenium (Li–Se) battery is an alternative to the lithium-sulfur battery which is used widely for storage energy.
- Selenium dithiocarbamate is used in processing natural and synthetic rubber.
- For making solar cells, we use copper indium gallium selenide.
Function of Selenium
It is a trace mineral that is not produced in the body but is required in many cellular functions. It is commonly controlled thyroid and immune system function in our bodies.
The element Se is an essential part of various enzymes and proteins such as selenoproteins. The main functions of selenium are,
- It can replace the amino acid residue of the protein to form selenoproteins. Selenoproteins can help to make DNA and the metabolism of thyroid hormones.
- The enzyme is probably effective in protecting lipids in the cell membrane.
- Selenium also protects animals or humans from carcinogenic chemicals. Therefore, it is functioning as a cancer prevention material. The element is able to bind toxic heavy metals such as cadmium and mercury.
- Vitamin E with selenium prevents the development of hepatic necrosis and muscular dystrophy.
Toxic Effects
Selenium is an essential element and deficiency causes several types of health problems in living organisms. An excessive amount may be toxic and cause selenosis.
In animals, continued intake of high-level selenium may cause weight loss, emotional disturbance, hair loss, impaired vision, respiratory failure, paralysis, and eventually death.