Electrochemical Cell Types
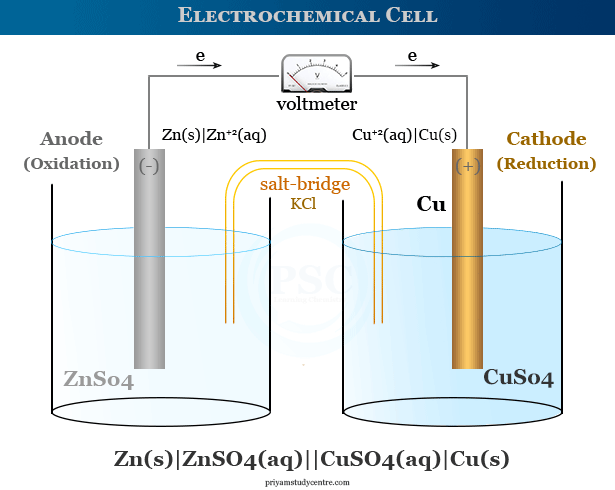
An electrochemical cell or simply a chemical cell is a device that produces electrical energy due to oxidation reduction or redox reactions. An electrolytic and galvanic cell are the two common types of electrochemical cells that produce electrical energy through an indirect redox reaction. The dry cell, fuel cell, and Daniell cell are common examples of electrochemical cells where chemical energy is converted to electrical energy. In the Daniell cell, a copper electrode is dipped into a copper sulfate solution and a zinc electrode is immersed in a zinc sulfate solution. Two components are separated by a porous barrier or salt bridge. In a Daniell cell, electrons are transferred from zinc atoms to copper ions. Electrochemical cells are also called Galvanic or Voltaic cells after the name of the scientists Luigi Galvani and Alessandro Volta.
Dry Cell Battery
A dry cell battery is an electrochemical cell that we use widely in various electronic gadgets. A dry cell battery contains an immobilized paste of electrolyte.
A zinc carbon battery is an example of a common dry cell in which a zinc plate is used as a negative electrode and a carbon rod is a positive electrode. Manganese dioxide (MnO2), ammonium chloride (NH4Cl), and zinc chloride paste are used as an electrolyte. Other examples of dry cell batteries that we use widely in our daily lives are,
Difference Between Electrolytic Cell and Galvanic Cell
An electrolytic and galvanic cell are the two common types of electrochemical cells. The main differences between an electrolytic cell and a galvanic cell are given below in the table,
| Galvanic cell | Electrochemical cell |
| A galvanic cell is a device in which the free energy of a chemical process is converted into electrical energy. | An electrolytic cell is one in which electric energy from an external source is used to perform a chemical change. |
| The redox reaction that takes place in a galvanic cell is spontaneous in nature. | Electrolysis of electrolytes that takes place in an electrolytic cell is non-spontaneous in nature. |
| The electrodes are placed in two different containers which contain different electrolytes connected by a salt bridge. | Both electrodes are placed in a single container that contains a single electrolyte in the form of a solution or molten state. |
| The anode acts as a negative charge electrode and the cathode acts as a positively charged electrode in the galvanic cell. | Similarly, the anode acts as a positive charge electrode and the cathode acts as a negatively charged electrode in the electrolytic cell. |
| The two electrodes are of different materials. | The two electrodes are of the same or different materials. |
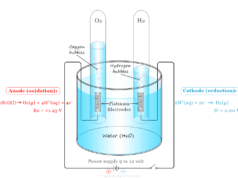
Anode and Cathode in Electrochemical Cell
An electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. An equation that describes the reaction at a single electrode of an electrochemical cell is called a half-cell reaction. Therefore, an electrode and the surrounding solution in the electrode components are called a half cell.
In the Daniell cell, the zinc electrode is the anode, and the copper electrode is the cathode. In the outer circuit, the electron flow occurs from zinc to copper.
Galvanic Cell or Voltaic Cell
A galvanic cell or voltaic cell is a commonly used cell in our daily life that helps to supply electric energy for different commercial purposes. There are mainly two types of galvanic or voltaic cells,
- Primary electrochemical cell
- Secondary electrochemical cell
Primary Electrochemical Cell
The primary cell is electrochemical cells where chemicals are used for the production of electrical energy. They cannot be brought back to their original state
Leclanche or Dry cell is a primary cell in which the zinc plate is a negative electrode while the carbon rod is a positive electrode. The zinc plate usually covers the outer part of the cell.
Secondary Electrochemical Cell
A secondary cell is also a chemical cell that we use mainly for the storage of electricity and purification of metals.
The chemicals used in secondary cells can be brought back to their original state by charging with current from an external source. Therefore, the cell again fits to supply energy as before. The process can be repeated over and over again.
Fuel Cell
A fuel cell is an electrochemical cell in which the chemical energy available from a fuel (hydrogen or methane) is converted to electrical energy by its oxidation. The cells produce low voltages but they are capable of supplying direct current for considerable periods. Fuel cells also provide power at a low cost and less attention is required for their maintenance.
The bye products obtained from the fuel cell are harmless and they cannot cause pollution of our environment. Therefore, they are used as a clean source of energy.
Uses of Electrochemical Cell
- A very useful application of electrochemical cells is commercial electroplating by electrolysis.
- We used a lead storage battery and a nickel storage battery for the storage of electrical energy.
- In our daily life, we also use different types of dry cell batteries in electronic gadgets like TV remotes, torchlight, etc.
- For the production of metal, we used an electrolytic cell. For example, we used it for the extraction of sodium from molten sodium chloride.
- Electrolytic purification of impure metals like copper, silver, aluminum, gold, and zinc is also done by electrochemical cells.
- The fuel cell is an example of an electrochemical cell that is used as a clean source of energy.