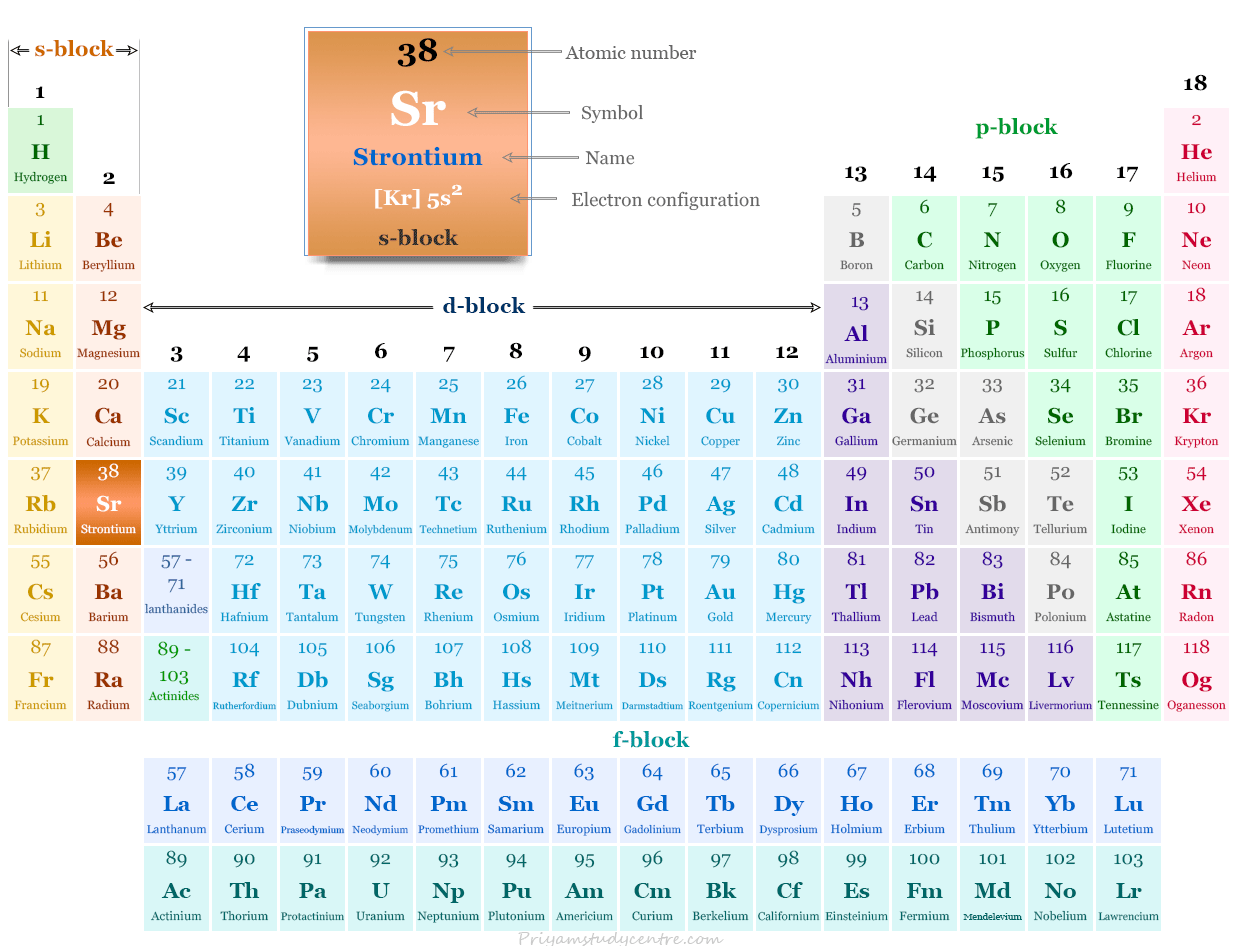
Strontium Element
Strontium is a chemical element or alkaline earth metal found in group 2 of the periodic table with the symbol Sr and atomic number 38. It is a silvery white, lustrous, soft metallic element. However, strontium is much harder than alkali metals due to the presence of two valence electrons for metallic bonding. It is chemically similar to that magnesium, calcium, and barium but more reactive than magnesium and calcium due to the larger size of the Sr atom. Like other alkaline earth metals, strontium also shows a +2 oxidation number or state. Strontium element was discovered in the ores of the lead mines found in the Scottish village of Strontian. It was discovered by the name of the Scottish village of Strontian. The metal was first isolated in 1808 by Humphry Davy using the process of electrolysis.

Where is Strontium Found?
Strontium is the 15th most abundant element on Earth found mainly in the minerals celestite (SrSO4) and strontianite (SrCO3). Due to high chemical reactivity, it only exists in nature when combined to form minerals. Like calcium, a very small concentration of Sr is found in groundwater and seawater.
Most of the strontium found in the human body is concentrated in our bones. The supplement SrCl2 is used in medicine for the treatment of tooth sensitivity, osteoporosis, osteoarthritis, and prostate cancer.
China is now the leading world producer of strontium but it may also be found in Spain, Mexico, Argentina, Morocco, and the United States.
Isotopes of Strontium
It has four stable natural isotopes 84Sr, 86Sr, 87Sr, and 88Sr. The abundance of these isotopes increases with increasing mass numbers. Beta decay of 87Rb gives pure 87Sr in some minerals. It is the basis of Rb-Sr dating.
89Sr and 90Sr are the two important isotopes of metal. In radiopharmaceuticals, 89Sr is used for the treatment of bone cancer.
90Sr is a highly radioactive isotope of strontium. Due to high-energy radiation, Sr-90 may be used to generate electric current for space vehicles, remote weather stations, and navigation buoys.
Properties
Strontium is a soft, silver-white, and highly chemically reactive metal that forms a dark oxide layer when it is exposed to air. It is harder than alkali metals due to the presence of two valence electrons for metallic bonding.
The physical and chemical properties of Sr are similar to that of vertical neighbors calcium and barium in the periodic table.
The crystal structure of strontium is similar to that of calcium. Both metals adopt face-centered cubic crystal lattices.
| Strontium | |||
| Symbol | Sr | ||
| Discovery | Adair Crawford in 1790 | ||
| Name derived from | Strontian means a small town in Scotland | ||
| Common isotopes | 38Sr86, 38Sr87, 38Sr88 | ||
| Oxidation states | +2 | ||
| CAS number | 7440-24-6 | ||
| Periodic properties | |||
| Atomic number | 38 | ||
| Relative atomic mass | 87.62 | ||
| Electron per cell | 2, 8, 18, 8, 2 | ||
| Electronic Configuration | [Kr] 5s2 | ||
| Block | s-block | ||
| Group | 2 | ||
| Period | 5 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 777 °C, 1050 K | ||
| Boiling point | 1377 °C, 1650 K | ||
| Molar heat capacity | 26.4 J mol−1 K−1 | ||
| Crystal structure | face-centered cubic (fcc) | ||
| Density | 2.64 g/cm3 | ||
| Electrical resistivity | 132 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.49 Å | ||
| Covalent radius | 1.90 Å | ||
| Electronegativity | 0.95 (Pauling scale) | ||
| Electron affinity | 4.631 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 549.47 | 1064.24 | 4138.26 | |
Strontium in the Periodic Table
It is found in group 2 and the period 5 of the periodic table. Strontium is an alkaline earth metal that lies between calcium and barium.
Extraction Process
Strontium is extracted mainly from the mineral celestine. The mined celestine (SrSO4) is converted to carbonate by two processes.
- In the first process, the celestine may be directly leached with sodium carbonate solution to form Strontium carbonate.
- In the second process, celestine is roasted with coal to form the dark-colored sulfide.
SrSO4 + 2 C → SrS + 2 CO2
It is called black ash. The black ash may dissolve in water and filter to obtain SrS. It can be converted to SrCO3 by the introduction of carbon dioxide.
Commercially, the metal is produced by reducing strontium oxide with aluminium.
3 SrO + 2 Al → 3 Sr + Al2O3
A small scale of Sr metal can also be prepared by electrolysis of a solution of strontium chloride in molten potassium chloride.
Sr2+ + 2 e → Sr
2 Cl− → Cl2 + 2 e
Chemical Compounds
The electron configuration of Sr is [Kr] 5s2. Therefore, Sr is characterized by two valence electrons in the 5s orbital outside the noble gas core. These two electrons are uniformly involved together to show uniform bivalency in strontium compounds.
The high value of the third ionization energy or closed shell configuration of Sr+2 ion also explains the upper limit of the +2 oxidation number or state. The most common compounds of Sr are given below,
Strontium Oxide
Strontium oxide or strontia is a strongly basic oxide with the chemical formula of SrO. Burning Sr metal in the air produces a mixture of oxides and nitride.
Strontia is formed when strontium reacts with oxygen. It is also obtained from the decomposition of SrCO3. It is used mainly for making glass in colour television cathode-ray tubes.
Hydroxide
Strontium hydroxide is a caustic alkali with the chemical formula Sr(OH)2. It exists in anhydrous, monohydrate, or octahydrate form.
It is obtained by combining a strontium salt with a strong base. Sr(OH)2 is used chiefly for refining beet sugar and stabilizing plastics.
Strontium Chloride
It is a salt of strontium and chlorine with the chemical formula SrCl2. It can be prepared by treating aqueous Sr(OH)2 or SrCO3 with hydrochloric acid.
Sr(OH)2 + 2 HCl → SrCl2 + 2 H2O
It is a starting material for the production of other strontium compounds such as SrCrO4, SrCO3, SrSO4, etc. It is also used in toothpaste for sensitive teeth, in biological research, and for storing ammonia solution.
Carbonate
It is a carbonate salt of Sr with the chemical formula SrCO3. A white or grey powder of carbonate is found in nature as the mineral strontianite. It is used commonly as an inexpensive colorant in fireworks.
SrCO3 is prepared synthetically by the reaction of SrS with carbon dioxide gas or sodium carbonate.
SrS + H2O + CO2 → SrCO3 + H2S
SrS + Na2CO3 → SrCO3 + Na2S
Strontium Acetate
It is a compound of strontium with the chemical formula Sr(C2H4O2)2. Like other acetates, it is a white solid that is soluble in water. It may be obtained by reaction of Sr(OH)2 or SrCO3 with acetic acid.
Uses of Strontium
The use of alkaline earth metal strontium is similar to that of calcium and barium but it is rarely used due to its high price. The most common uses may include,
- It was used for making glass for colour television cathode-ray tubes. Presently, such applications are gradually disappearing due to the rising popularity of tubeless LCD and plasma TV sets.
- Small amounts of Sr are used for refining zinc metal. It is used mainly for removing lead impurities.
- Like barium, it is also used to remove unwanted gases in vacuums.
- Chemically and biologically inert strontium aluminate is used to glow the dark toys.
- SrCO3 and other salts are added to fireworks in order to create red colors.
- Carbonate salt is also used for making hard ferrite magnets.
- Strontium chloride hexahydrate is an ingredient that is used in toothpaste for sensitive teeth.
- In radiopharmaceuticals, the radioactive isotope 89Sr is used for treating bone cancer.
- The other radioactive isotope, 90Sr is a useful by-product of nuclear reactors. The high-energy radiation of strontium-90 may be used to generate electric current for space vehicles, remote weather stations, and navigation buoys.
Strontium in the Human Body
It is an important trace element in the human body that is found mainly in bone and related tissues. It has a significant role in bone metabolism.
In our body, Sr behaves like calcium. In adults, it mostly attaches to the surfaces of bones. The children whose bones are still growing, strontium may be used to increase the hardness of bone.