Barium Element
Barium is a chemical element or alkaline earth metal found in group 2 of the periodic table with the symbol Ba and atomic number 56. Elemental barium is a soft, silvery metal that is never found in nature as a free element and the softness of the element is lower than that of alkali metals due to the presence of two valence electrons for metallic bonding. It is chemically similar to magnesium, calcium, and strontium but more reactive than magnesium and calcium due to the larger size of the Ba atom. Like other alkaline earth metals, barium also shows a +2 oxidation state or number. It was identified in 1774 but not obtained until 1808. In 1808, Sir Humphry Davy first isolated Ba by electrolysis of molten barium salt. The name of the element is derived from the Greek word barys means heavy.

Where is Barium Found?
Barium is found mostly in the minerals barytes or heavy spar (BaSO4) and witherite (BaCO3). The primary commercial source, baryte or barium sulfate is deposited in many parts of the world. Another commercially less important source is witherite or barium carbonate.
The main deposits and produced countries are located in Britain, Romania, China, India, Morocco, the US, Turkey, Iran, and Kazakhstan.
A small amount of barium is also found in seawater as Ba+2 ions and BaSO4, or barite. The constant concentration of Ba is seen in the upper ocean seawater except in the region where the river goes to the ocean.
Production Process
Robert Bunsen and Augustus Matthiessen can produce pure Ba metal by electrolysis of a molten mixture of barium chloride and ammonium chloride. Commercially, it can be produced by the following process,
- The mined baryte ore is washed, crushed, and separated from quartz. If the concentration of iron, zinc, or lead content is abnormally high, the froth flotation process is used. It formed 98% pure baryte.
- 98% pure baryte then reduced by carbon to form barium sulfide.
BaSO4 + 2 C → BaS + 2 CO2 - The sulfide can be treated with nitric acid to give barium nitrate.
- The nitrate can be thermally decomposed to yield BaO.
- The reduction of BaO in an evacuated retort with aluminum gives the best way for the production of metal Ba.
2 Al + 3 BaO → 3 Ba + Al2O3
Silicon is also used as a reducing agent for the reduction of BaO at 1200 °C.
Si + 3 BaO → 2 Ba + BaSiO3
BaS is also a starting point for the production of other compounds like barium sulfate and carbonate. When BaS is treated with oxygen, it gives sulfate but when treated with carbon dioxide, it gives barium carbonate.
Properties
Barium is a soft, silvery-white metal that forms a slight golden shade in an ultrapure state. It is harder than alkali metal due to the presence of two valence electrons for metallic bonding. The melting points of alkaline earth metals are considerably higher than alkali metals for the same reason.
The silvery-white color of Ba metal rapidly vanishes in the air due to the formation of a dark gray layer containing the BaO. Like radium, it also adopts a body-centered cubic crystal structure.
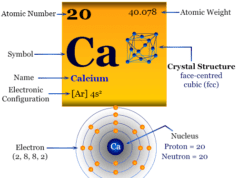
| Barium | ||
| Symbol | Ba | |
| Discovery | Humphry Davy in 1808 | |
| Name derived from | The Greek word barys means heavy | |
| Common isotopes | 88Ra138 | |
| Oxidation states | +2 | |
| CAS number | 7440-39-3 | |
| Periodic properties | ||
| Atomic number | 56 | |
| Relative atomic mass | 137.327 | |
| Electron per cell | 2, 8, 18, 18, 8, 2 | |
| Electronic Configuration | [Xe] 6s2 | |
| Block | s-block | |
| Group | 2 | |
| Period | 6 | |
| Physical properties | ||
| State at 20 °C | Solid | |
| Melting point | 727 °C, 1000 K | |
| Boiling point | 1845°C, 2118 K | |
| Molar heat capacity | 28.07 J mol−1 K−1 | |
| Crystal structure | face-centered cubic (fcc) | |
| Density | 3.62 g/cm3 | |
| Electrical resistivity | 332 nΩ m | |
| Atomic properties | ||
| Atomic radius (non-bonded) | 268 Å | |
| Covalent radius | 2.06 Å | |
| Electronegativity | 0.89 (Pauling scale) | |
| Electron affinity | 13.954 kJ mol−1 | |
| Ionization energy (kJ/mol) | 1st | 2nd |
| 502.85 | 965.22 | |
All alkaline earth metals are characterized by two valence electrons in an s-shell outside the noble gas core. These two electrons are always involved together to give uniform bivalency to these elements.
The closed shell configuration of the Ba+2 ion explains the upper limit of the +2 oxidation number or state of barium. At room temperature, it reacts with oxygen or air. Therefore, it may be stored in an oil or inert atmosphere.
Barium in the Periodic Table
Barium is found in group 2 and period 6 of the periodic table. It is an alkaline earth metal or s-block element that lies between strontium and radium.
Facts About Ba
- The metal barium reacts with nonmetals such as carbon, nitrogen, phosphorus, silicon, and hydrogen by producing heat.
- It may also react exothermally with water and alcohol by releasing hydrogen gas.
- The metal may readily react with mineral acids like nitric or hydrochloric acid. It may not react readily with sulfuric acid due to the formation of an insoluble barium sulfate layer.
- Ammonia may react with Ba metal to form Ba(NH3)6 complex.
- It can combine with other metals such as aluminium, zinc, lead, and tin to form various types of alloys.
Chemical Compounds
The valence shell electronic configuration of Ba is [Xe] 6s2. The element is characterized by two valence electrons in the 6s-orbital outside the noble gas core. These two electrons are always involved together to show uniform bivalency in barium compounds.
The most common chemical compounds of barium are given below,
Barium Oxide
Barium oxide or baria is a white hygroscopic non-flammable chemical compound with the formula of BaO. It is produced by heating barium carbonate with coke or by thermal decomposition of barium nitrate. BaO is used mainly in cathode ray tubes, crown glass, and catalysts.
It is toxic to human skin and causes irritation. The common side effects of barium oxide are nausea, diarrhea, muscle paralysis, and cardiac arrhythmia. It may also cause death.
Barium Hydroxide
Barium hydroxide with the chemical formula Ba (OH)2 can be obtained by treating BaO with water or by adding Ba to an aqueous solution.
BaO + H2O → Ba(OH)2
It absorbs very little carbon dioxide from aqueous solutions. This property is used for the calibration of the pH meter.
Barium Chloride
It is manufactured by strongly heating a mixture of BaSO4, coke, and calcium chloride.
BaSO4 + 4 C + CaCl2 → BaCl2 + CaS + 4 CO
The mass is extracted with water, concentrated, and crystallized. Some calcium sulfide may be retained in the solution. It may be precipitated by the addition of little lime.
Barium Sulfate
Barium sulfate is a white crystalline solid that has the chemical formula BaSO4. It is insoluble in water and obtained commercially from the mineral barite. Due to the insolubility of BaSO4, it is non-toxic for human beings.
BaSO4 is used mainly in drilling fluids, radiocontrast agents, pigment, heat-reflecting paint, paper brighteners, plastic fillers, etc.
Lithopone
Lithopone is a brilliant white pigment obtained by mixing BaS and ZnSO4 solution.
BaS + ZnSO4 → BaSO4 + ZnS
It is used widely in paints, inks, leather, paper, linoleum, and face powder. Lithopone does not blacken in atmospheric hydrogen sulfide. But it is slightly dark in light due to the liberation of metallic zinc.
Uses of Barium
The alkaline earth metal, barium has few industrial applications. The common uses of barium are,
- It is alloyed with aluminium and removes unwanted gases or gettering from vacuum tubes such as TV picture tubes. Presently, such applications are gradually disappearing due to the rising popularity of tubeless LCD and plasma TV sets.
- A Ni-Ba alloy has high emissive power and is used for spark-plug wire.
- It is alloyed with calcium, manganese, silicon, and aluminium to give high-grade steel deoxidizers.
- Barium sulfate (BaSO4) is an important chemical compound for the petroleum industry. It is used for drilling fluid in oil and gas wells.
- BaSO4 is used as a radiocontrast agent in X-ray imaging of the digestive system due to its low toxicity and relatively high density.
- BaO has been used for coating the electrodes of fluorescent lamps and facilitates the release of electrons.
- Many barium compounds are used to make paint, bricks, tiles, glass, and rubber.
Toxicity of Barium
Toxicological data on barium metal can be found due to its high reactivity. The data is only available for Ba compounds. BaSO4 is non-toxic in nature but soluble chemical compounds are poisonous.
The low doses of Ba+2 ion cause muscle stimulants but high doses affect the nervous system, causing cardiac irregularities, tremors, weakness, anxiety, shortness of breath, and paralysis. Water-soluble barium compounds affect mainly our eyes, immune system, heart, respiratory system, and skin. They cause blindness and irritation to the skin.
Due to high reactivity, it is always stored in an argon atmosphere or under mineral oils. In air or oxygen, it may cause ignition. We always handed barium or its compounds with non-sparking shoes, flame-resistant rubber clothes, rubber gloves, an apron, goggles, and a gas mask.