Radium Metal
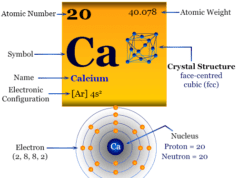
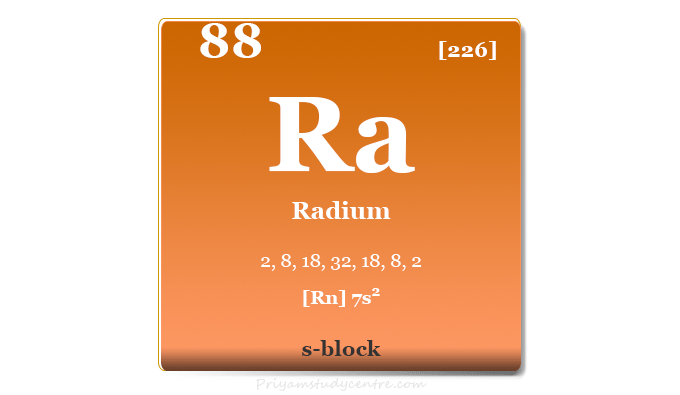
Radium is a chemical element or radioactive metal found in group 2 of the periodic table with the symbol Ra and atomic number 88. Pure radium is a silvery-white alkaline earth metal that readily reacts with nitrogen to form a black layer of radium nitride. All four isotopes of radium metal are radioactive in nature and the most stable isotope, Ra-226 has a half-life of 16 years. Ra does not occur naturally in significant amounts and it occurs in small amounts in pitchblende. Ra is a radioactive decay product of uranium and thorium decay chains. The compound radium chloride was discovered by Marie and Pierre Curie in 1898 from uranium ore uraninite. In 1911, the metallic form of radium was extracted by Marie Curie and André-Louis Debierne through the electrolysis of radium chloride.
Where is Radium Found?
Trace levels of radium are found in our natural environments such as rock, soil, water, plants, and animals. The occurrence of Ra in Earth’s crust and sea water is very small. One kilogram of Earth’s crust contains only 900 picograms of Ra and one liter of sea water contains only about 89 femtograms of Ra.
Radium is recovered in small quantities as a by-product of uranium refining. It may be produced in less than 100 g a year over the world. Nearly 10 tonnes of ore have to be used for the production of 1 mg of Ra.
Isotopes
Radium has 33 known radioactive isotopes with mass numbers ranging from 202 to 234. Four of these isotopes occur naturally in the thorium-232, uranium-235, and uranium-238 decay chains. The half-life of these isotopes,
- 223Ra: half-life 11.4 days
- 224Ra: half-life 3.64 days
- 226Ra: half-life 1600 years
- 228Ra: half-life 5.75 years
The most common isotope of Ra is 226Ra with a half-life of 1600 years. It occurs in the decay chain of 238U.
How is Radium Extracted?
With minor modification, the extraction process of radium from pitchblende remains the same as that used by Marie and Pierre Curie.
- Pitchblende is finely grounded and roasted over 600 °C.
- It is digested with 1:1 sulfuric acid for six hours. Uranium, iron, manganese, and copper go into the solution. Radium remains insoluble as sulfate with BaSO4, PbSO4, silica, etc.
- The residue is leached with sodium thiosulfate solution to remove silver and digested with 10% sodium hydroxide for removal of lead and silica.
- The sulfates are then converted to carbonate by reaction with Na2CO3. The carbonate is dissolved in hydrochloric acid to give chlorides. These two processes are repeated several times.
- Finally, the carbonate is dissolved in HBr to give RaBr2 and BaBr2.
- Less soluble RaBr2 may be separated from BaBr2 by fractional crystallization. 20 to 200 crystallization may be necessary to obtain sufficient pure RaBr2.
- The product is ignited at 600 °C and sealed with glass capillaries. It is usually supplied in this form.
- Metallic radium may be obtained by electrolysis of RaBr2 or RaCl2 solution.
Properties
Radium is a silvery white, lustrous, soft, radioactive metal that contains two valence electrons for metallic bonding. Due to the presence of two valence electrons for metallic bonding, alkaline earth metals are relatively harder than alkali metals.
| Radium | ||
| Symbol | Ba | |
| Discovery | Pierre and Marie Curie in 1898 | |
| Name derived from | The Latin word radius means ray | |
| Common isotopes | 56Ba226 | |
| Oxidation number or state | +2 | |
| CAS number | 7440-14-4 | |
| Periodic properties | ||
| Atomic number | 88 | |
| Relative atomic mass | [226] | |
| Electron per cell | 2, 8, 18, 32, 18, 8, 2 | |
| Electronic Configuration | [Rn] 7s2 | |
| Block | s-block | |
| Group | 2 | |
| Period | 7 | |
| Physical properties | ||
| State at 20 °C | Solid | |
| Melting point | 696° C, 969 K | |
| Boiling point | 1500 °C, 1773 K | |
| Natural occurrence | from radioactive decay | |
| Crystal structure | face-centered cubic (fcc) | |
| Density | 5 g/cm3 | |
| Electrical resistivity | 1 µΩ m | |
| Atomic properties | ||
| Atomic radius (non-bonded) | 2.83 Å | |
| Covalent radius | 2.11 Å | |
| Electronegativity | 0.90 (Pauling scale) | |
| Electron affinity | 9.65 kJ mol−1 | |
| Ionization energy (kJ/mol) | 1st | 2nd |
| 509.29 | 979.05 | |
Radium in the Periodic Table
It is placed in group 2 and period 7 of the periodic table. Radium is an alkaline earth metal that lies with beryllium, magnesium, calcium, strontium, and barium.
Facts About Radium
- It is harder than alkali metals due to the availability of two electrons for metallic bonding.
- The melting points of alkaline earth metals are considerably higher for the same reason.
- Like other alkaline earth metals, it is a highly reactive metal and always shows an oxidation state of +2.
- It forms colorless Ra2+ cation in an aqueous solution which is highly basic in nature.
- The high value of third ionization energy is quite expected from the closed shell configuration of Ra+2 ion. It explains the upper limit of the +2 oxidation state of elements.
- Most of the radium compounds are ionic in nature.
- The standard electrode potential for the half-reaction is slightly lower than the preceding metal barium. Generally, the reduction potential of group 2 elements increased down the group.
Uses of Radium
Presently, the use of radium may be reduced due to its high radioactivity.
- The form of radium like RaCl or RaBr uses in medicine to produce radon gas. It was used previously to kill the cancerous cells present in bones. Presently it is replaced with safer gamma emitters 60Co because 60Co is less costly and available in larger quantities.
- Formerly it was used in luminous paints for watches, nuclear panels, aircraft switches, clocks, etc.
- At the beginning of the 19th century, it was used as an additive in toothpaste, hair creams, and food products. The use of radium in such items is now strictly prohibited due to its serious health effects and radioactive nature.
- Radium acts as a neutron source for nuclear power reactors when mixed with beryllium.
- It is used in the field of atomic, molecular, and optical physics research.
Health Effects
It is highly radiotoxic and carcinogenic in nature. It is more radioactive than uranium metal. Inhalation of radium may increase the risk of cancer, particularly lung and bone cancer, and causes various serious health problems. A higher level of radium may cause anemia and bone cancer which reduces the growth of our bones.
Exposure to Ra from internal or external sources can cause cancer and other health disorders. Radium and radon emit alpha and gamma rays upon their radioactive disintegration which kill or mutation of cells in our bodies.