Lanthanum Element
Lanthanum is the f block element or rare-earth metal of the periodic table with atomic number 57 and symbol La. It is a soft, silvery-white rare earth metal that rapidly tarnishes in the air and burns easily when heated. The valence shell electronic configuration of La is [Xe] 5d1 6s2 4f0. The name rare earth was given because they were originally extracted from rare earth oxide. It was first discovered by the Swedish chemist Carl Gustaf Mosander in 1839 and the name lanthanum was given from the Ancient Greek latter lanthanein which means to lie hidden. It is the first f-block element and prototype of the lanthanide family. On heating, lanthanum reacts with a number of nonmetals like nitrogen, sulfur, silicon, phosphorus, hydrogen, etc. It also forms salt-like hydride (LaH3 and LaH2) which contains H− ion in the compound.

Properties of Lanthanum
Lanthanum is part of the scandium group or group 3 (IIIA) due to the presence of (n − 1) d1 ns2 electronic configuration. Due to the lack of common features of d-block elements, it shows a single stable oxidation number or state like +3, and closely resembles lanthanides.
| Lanthanum |
|||
| Symbol | La | ||
| Discovery | Discovered by Carl Gustav Mosander in 1839 | ||
| Name derived from | The Greek word lanthanein means to lie hidden | ||
| Common isotope | 57La139 | ||
| CAS number | 7439-91-0 | ||
| Periodic properties | |||
| Atomic number | 57 | ||
| Atomic weight | 138.905 | ||
| Electron per shell | 2, 8, 18, 18, 9, 2 | ||
| Electronic configuration | [Xe] 5d1 6s2 | ||
| Block | f-block | ||
| Period | period 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 920 °C or 1688 °F | ||
| Boiling point | 3464 °C or 6267 °F | ||
| Density | 6.162 g/cm3 | ||
| Molar heat capacity | 27.11 J mol−1 K−1 | ||
| Electrical resistivity | 615 nΩ m | ||
| Chemical properties | |||
| Atomic radius (non-bonded) | 2.43 Å | ||
| Covalent radius | 1.94 Å | ||
| Oxidation number | +3 | ||
| Electronegativity | 1.10 (Pauling scale) | ||
| Electron effinity | 45.35 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 538.1 | 1067 | 1850.3 | |
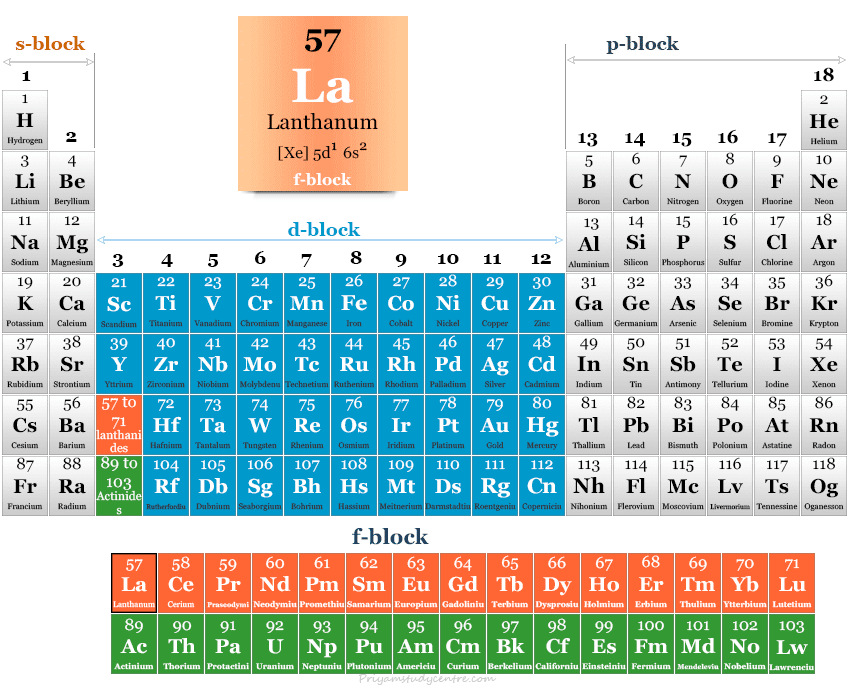
Lanthanum in the Periodic Table
Lanthanum is a rare earth element placed within the f-block element in the periodic table.
Where is Lanthanum Found?
Lanthanum is the third-most abundant of all the lanthanides. There are 200 minerals known which contain rare earth metals.
The two most commercially important minerals are monazite and bastnaesite. Monazite is a mixed phosphate of lanthanum, cerium, thorium, and other rare-earth. Bastnaesite is a fluoride carbonate of lanthanum and other rare earth metals, but the heavier elements are virtually absent from it.
Naturally, occurring lanthanum contains two isotopes 139La and 138La. 139La is most abundant but 138La is rare.
Monazite is chemically quite inert and has a high density. It is mostly concentrated in beach sands and river beds through weathering.
It is found in southern India, South Africa, Brazil, Australia, and Malaysia. Generally, we used monazite for the extraction of lanthanum and other rare earth metals.
Production Process
The main steps involved in the production of lanthanum and other rare earths are as follows:
- Digestion of the concentrated ore by concentrated aqueous sodium hydroxide (NaOH) at 140 °C, followed by extraction with water.
- The slurry of impure hydrous oxides is boiled with hydrochloric acid around a pH scale of 3.5. ThO2 is precipitated and filtered out.
- The filtrate containing LaCl3 and LnCl3 (Ln = lanthanides) is treated with a solution of BaCl2.
- A stoichiometric amount of Ln2(SO4)3 is used to precipitate the entire barium as BaSO4. BaSO4 carries traces of RaSO4 which are removed by filtration.
- The filtrate contains LaCl3 and LnCl3 from which La may be separated by fractionation crystallization.
However, the most modern methods for the extraction of lanthanides are solvent extraction and ion exchange chromatography techniques.
Chemical Properties
Lanthanum is silvery-white, soft, and malleable with high electrical conductance. It is highly electropositive and reactive. Therefore, the reactivity of higher members of lanthanides is comparable to calcium and scandium to aluminum.
The compact metal is quite stable to dry air at ordinary temperatures. It burns in the air to form oxide (La2O3) and nitride (LaN).
Uses of Lanthanum
- The rare earth metal, lanthanum has no commercial uses. It is used as an additive to steel to improve strength and workability.
- It is used for the preparation of mischmetal. However, due to the high production cost of lanthanum, we used mixed lanthanide for the preparation of mischmetal.
- Hydrogen sponge alloys can contain lanthanum which is used for storing hydrogen gas in power vehicles.
- It is also used for manufacturing solid oxide batteries.
- Lanthanum or rare earth metal compounds are widely used in photo studios and projectors because these compounds increase the brightness of the surface.
- Lanthanum trifluoride (LaF3) is used for the production of heavy fluoride glasses. For example, it is used in optical fiber for communication.
- La(III) oxide (La2O3) is used generally for the production of optical glasses. It is used in camera and telescope lenses. Optical glass is also used for storing alkali metals due to its high alkali resistance properties.
- Mixed lanthanides containing lanthanum are added to zeolite to enhance the chemical catalyst activity. It is used in the creaking of petroleum.