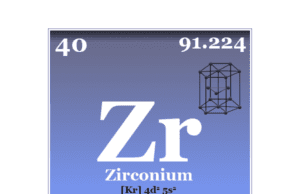
Scandium Element
Scandium is a chemical element or transition metal of Group 3 of the periodic table with atomic number 21 and symbol Sc. The common oxidation number or state of scandium is +3 due to the presence of three electrons in its outer orbital. Sc is a silvery-white, moderately soft metal that usually occurs with lanthanides due to the lack of the characteristics of transition metals. The chemistry or chemical properties of scandium resemble the metal aluminum. Therefore, Russian chemist Dmitry Ivanovich Mendeleyev named it ekaboron. The silvery-white hexagonal crystal lattice, Sc has the valence shell electronic configuration [Ar] 3d1 4s2. The common oxidation number or state of the metal is +3 with no d-electron.
Where is Scandium Found?
It is the 50th most abundant element in the earth’s environment and the 23rd most abundant element in the Sun due to its high cosmic abundance.
In the earth’s crust, scandium is not rare, it is found with the lanthanides and uranium ore (0.02 percent of Sc2O3) which may be obtained as a by-product. Thortveitite (Sc2SiO7) is a mineral of scandium found mostly in Norway.
Scandium on the Periodic Table
Scandium, yttrium, and lanthanum constitute group 3 of the periodic table. They are the beginning members of the first three transition series. They have a d1 s2 electronic configuration over a closed noble gas shell.
Properties of Scandium
Some physical and chemical properties of scandium are given below in the table,
| Scandium | |||
| Symbol | Sc | ||
| Discovery | Lars Frederik Nilson in 1879 | ||
| Name derived from | From Scandia, the Latin name for Scandinavia | ||
| Common isotope | 45Sc | ||
| Oxidation states | 0, +1, +2, +3 | ||
| CAS number | 7440-20-2 | ||
| Periodic properties | |||
| Atomic number | 21 | ||
| Relative atomic mass | 44.956 | ||
| Electron per cell | 2, 8, 9, 2 | ||
| Electronic Configuration | [Ar] 3d1 4s2 | ||
| Block | d-block | ||
| Group | 3 | ||
| Period | 4 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1541 °C, 2806 °F, 1814 K | ||
| Boiling point | 2836 °C, 5137 °F, 3109 K | ||
| Molar heat capacity | 16.443 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 25.52 g/cm3 | ||
| Heat of fusion | 14.1 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.15 Å | ||
| Covalent radius | 1.59 Å | ||
| Electronegativity | 1.36 (Pauling scale) | ||
| Electron affinity | 18.139 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 633.09 | 1234.99 | 2388.65 | |
Production Process
Scandium minerals may be treated similarly to bauxite to obtain insoluble ScO(OH) and dehydration of hydrous oxide to form Sc2O3.
The metal is obtained by the electrolysis of a molten mixture of scandium trichloride (ScCl3), potassium chloride (KCl), and lithium chloride (LiCl).
Isotopes of Element
In nature, 45Sc is the most stable isotope of scandium. It also has thirteen unstable short-lived radioactive isotopes with atomic masses ranging from 36 to 61.
46Sc is the most stable radioactive isotope (half-life of 83.8 days) and 39Sc is the least stable isotope with a half-life of fewer than 300 nanoseconds.
Primarily, calcium or titanium isotopes are formed by the radioactive decay of scandium isotopes. Atomic mass below 46 primarily formed calcium isotopes while above 46 formed titanium isotopes by nuclear reaction.
Facts About Scandium
- The chemistry and facts of scandium resemble very closely the element aluminum. Both metals are dominated by the +3 state or form trivalent ions like Al+3 and Sc+3.
- Sc is less electropositive than the preceding group member calcium.
- It is a transition element due to the presence of a d electron. However not many of the properties (variable oxidation number) associated with Sc. Therefore, it behaves more like non-transition metals.
- Sc dissolves in both acids and alkalis but does not react with the 1:1 mixture of nitric acid and hydrofluoric acid due to the formation of protective layers.
- Nitrogen combines with it at high temperatures to form ScN, which on hydrolysis by water.
Chemical Compounds
Scandium Halide
Halogens like fluorine, chlorine, and bromine react with scandium to form ionic halides (ScX3). ScF3 is the only insoluble halide that is dissolved in ammonium fluoride or alkali metal fluoride to give octahedral [ScF6]−3 ions.
The trichloride like ScCl3 is isostructural with AlCl3. The reaction of ScCl3 with excess Sc at high temperatures gives lower halides (ScCl) which contain a layer structure.
Scandium Oxide
Sc (III) oxide or scandia is an oxide of metal that has the chemical formula Sc2O3. It is amphoteric in nature. Sc2O3 is dissolved in acids to give Sc (III) salts. In excess sodium hydroxide, it gives Na3[Sc(OH)6]−3.
Uses of Scandium
- Scandium alloy is strong like titanium and light like aluminum and is used for the production of the components of aerospace, sports equipment, baseball bats high performing materials, and bicycle frames.
- The alloy is used in Russian military aircraft like MIG-21 and MiG-29.
- The radioactive isotope 46Sc is also used as a tracer in the oil refinery process.
- It is widely used in the television industry.
- In the United States and major industrial-based countries, scandium halide lamps are used mostly for the production of highly efficient light like sunlight.