Platinum
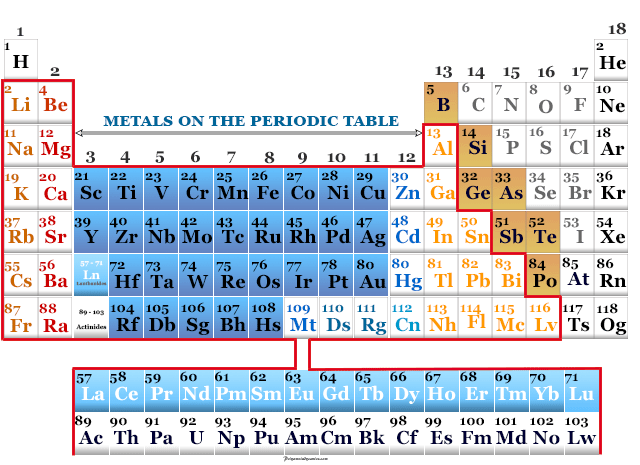
Platinum Metal
Platinum is a chemical element or silvery white, lustrous, malleable, high density metal of group-10 of the periodic table with atomic number 78...
Tungsten
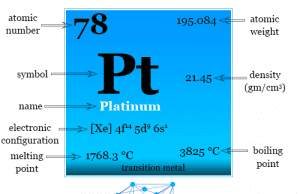
Tungsten Metal
Tungsten is a chemical element or strong, silvery transition metal of Group 6 (VIB) of the periodic table with the symbol W and atomic...
Molybdenum
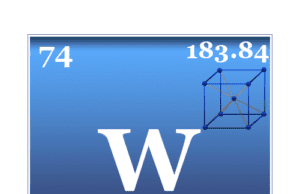
Molybdenum Metal
Molybdenum is a chemical element or strong and silvery transition metal of Group 6 (VIB) of the periodic table with the symbol Mo and...
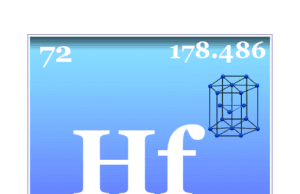
Hafnium
Hafnium Element
Hafnium is a chemical element or silvery-white transition metal of Group 4 (IVB) of the periodic table with the symbol Hf and atomic number...
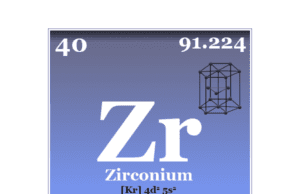
Zirconium
Zirconium Metal
Zirconium is a chemical element or silvery-white, hard, and high melting transition metal of Group 4 (IVB) of the periodic table with the symbol...
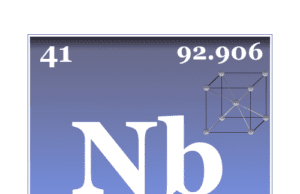
Niobium
Niobium Element
Niobium is a chemical element or light grey, crystalline, transition metal of Group 5 (VB) of the periodic table with symbol Nb and atomic...
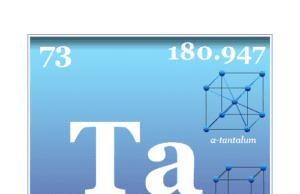
Tantalum
Tantalum Element
Tantalum is a chemical element or rare, hard, blue-gray, lustrous transition metal of Group 5 (VB) of the periodic table with the symbol Ta...
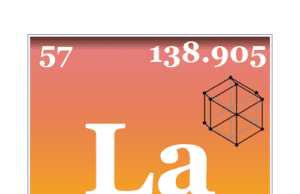
Lanthanum
Lanthanum Element
Lanthanum is the f block element or rare-earth metal of the periodic table with atomic number 57 and symbol La. It is a...
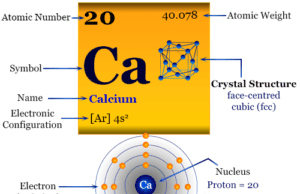
Calcium
Calcium Element
Calcium is the chemical element or alkaline-earth metal of Group-2 (IIA) of the periodic table with the symbol Ca and atomic number 20. It is...
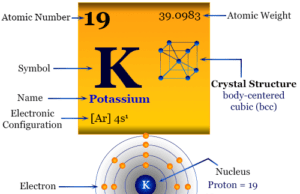
Potassium
Potassium Metal
Potassium is a chemical element or silvery-white alkali metal of Group-1 or IA of the periodic table with the symbol K and atomic number...