Beryllium Element
Beryllium is a chemical element or alkaline-earth metal of Group 2 (IIA) of the periodic table with the symbol Be and atomic number 4. It is used largely in metallurgy to improve the properties of copper and nickel alloys. It is the first member of alkaline earth metals with a very small size and very high effecting nuclear charge. The metal beryllium was discovered from the mineral beryl in 1798 (Vauquelin) but its atomic weight was finalized much later by Mendeleev. It was prepared independently by Wohler and Bussy in 1828 by the reduction of BeCl3 with potassium. Be metal forms a hexagonal closed-packed crystal lattice (HCP). It also shows a dipositive oxidation number or state by the liberation or sharing of two valence electrons. The first ionization energy is rather low than the second ionization energy of the beryllium atom.

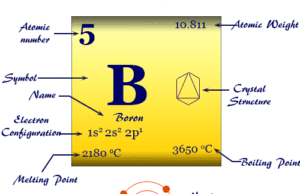
Properties of Beryllium
| Beryllium | |||
| Symbol | Be | ||
| Discovery | Nicholas Louis Vauquelin in 1797 | ||
| Name derived from | The Greek word for beryl means beryllo | ||
| Common isotope | 2Be9 | ||
| Oxidation states | 0, +1, +2 | ||
| CAS number | 7440-41-7 | ||
| Periodic properties | |||
| Atomic number | 4 | ||
| Relative atomic mass | 9.012 | ||
| Electron per cell | 2, 2 | ||
| Electronic Configuration | [He] 2s2 | ||
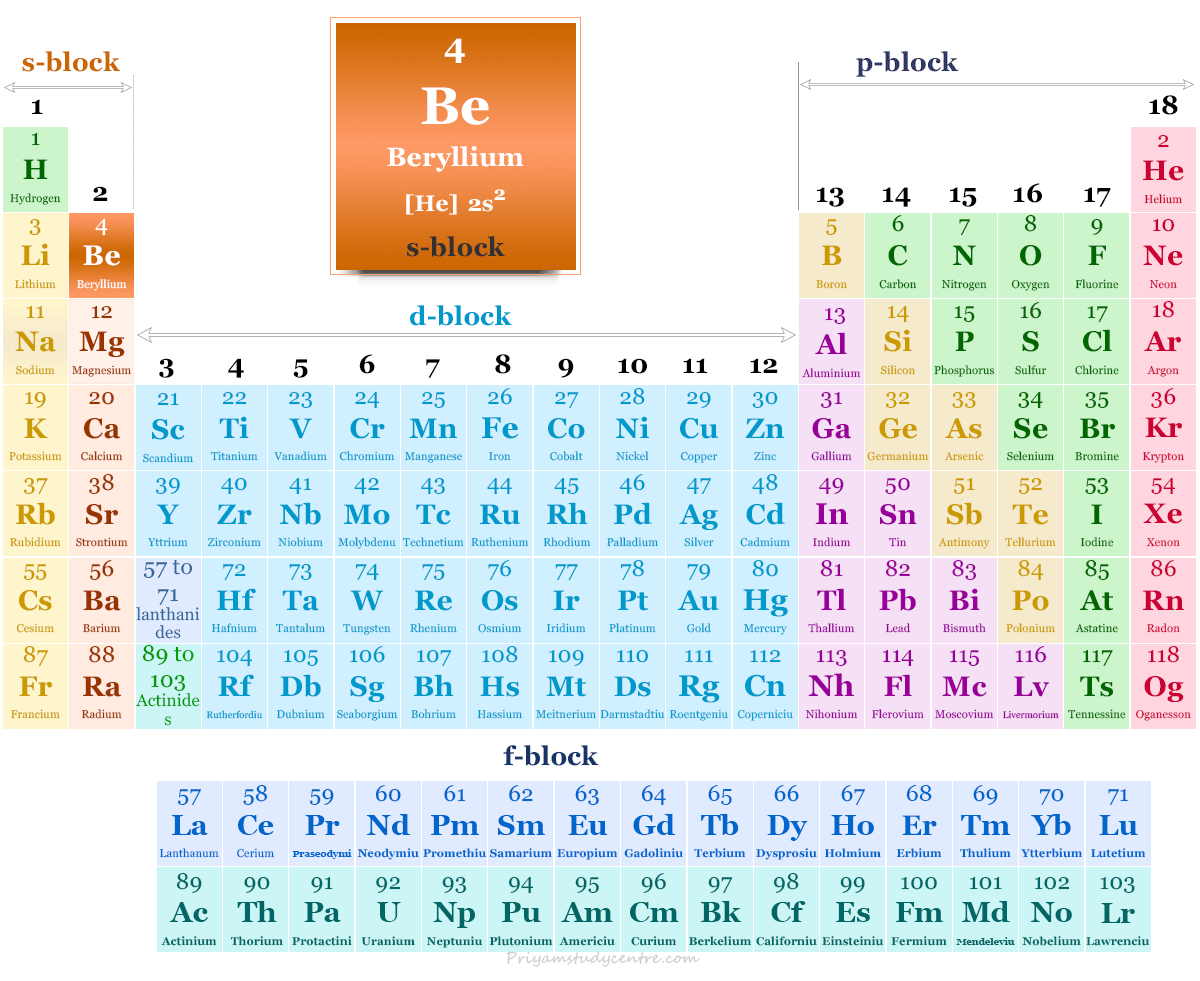
| Block | s-block | ||
| Group | 2 | ||
| Period | 2 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1287 °C, 2349 °F, 1560 K | ||
| Boiling point | 2468 °C, 4474 °F, 2741 K | ||
| Molar heat capacity | 16.443 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 1.85 g/cm3 | ||
| Electrical resistivity | 36 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 1.53 Å | ||
| Covalent radius | 0.99 Å | ||
| Electronegativity | 1.57 (Pauling scale) | ||
| Electron affinity | unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 899.50 | 1757.11 | 14848.77 | |
Beryllium in the Periodic Table
The alkaline earth metal beryllium is placed in period 2 and group 2 of the periodic table. It is an s-block element that lies with magnesium and calcium.
Where is Beryllium found?
The concentration of beryllium is very low (2 to 6 ppm) on the earth’s crust. The small abundance of beryllium is due to the transmutation of the Be atom under the natural bombardment of protons.
The element is readily accessible through the minerals like Beryl (Be3Al2Si6O18) and Phenacite (Be2SiO4). It is also found in pegmatite rocks and forms large beautiful crystalline solids that are used as gems like emeralds (green colour) or aquamarine (bluish-green colour).
Production Process
- The mineral beryl was roasted with sodium fluoride and Na2SiF6 at 700 °C to 750 °C to form insoluble Na3AlF6 and soluble Na2BeF4.
- The roasted mass is leached with boiling water to form a sodium fluoberyllate solution.
- The extract is precipitated as Be(OH)2 by sodium hydroxide at a pH scale of 12.
- The precipitate reacts with NH4HF2 to form ammonium fluoberyllate and is heated at 900°C to form BeF2.
- The metal beryllium is produced during the reduction of BeF2 with magnesium at 1300°C.
Chemical Properties of Beryllium
Beryllium (4Be8) has atomic number 4 and mass number 8. Therefore, Be nucleus contains 4 protons and 4 neutrons surrounded by four electron particles.
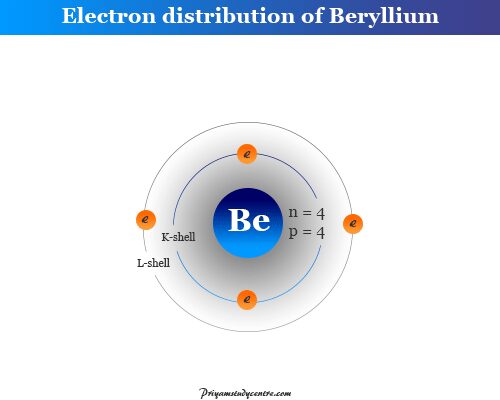
All the elements Group-2 like beryllium, magnesium calcium, strontium, barium, and radium are characterized by two valence electrons in the outermost s-shell. Therefore, these two electrons are always involved together in the formation of chemical bonding in chemistry.
The melting points of alkaline earth metals are considerably higher than that of alkali metals due to the variation of the crystal structure.
We observed that beryllium is much less reactive than the other elements in the group due to its small size. It dissolves in aqueous mineral acids like hydrochloric acid, sulfuric acid, and nitric acid to liberate hydrogen gas.
Interesting Facts About Beryllium
The charge-by-radius ratio for Be+2 is very high, greater than that of any cations except H+ and B+3. The closest value is found with Al+3. This fact suggests that beryllium and aluminum have similar physical and chemical properties.
- Both the metal generally dissolves in aqueous alkali by evolving hydrogen.
- The standard electrode potentials of the two metals are equal (−1.7 V).
- The oxides and hydroxides are amphoteric and the anhydrous halides of both metals are formed in dimeric compounds with metal-halogen-metal bridges.
- The thermal stability of both metal sulfates is nearly the same.
Chemical Compounds
Hydrides, Oxides, and Hydroxide of Beryllium
It does not form any hydrides directly but BeH2 may be obtained by reducing BeCl2 with lithium hydride with LiAlH4. Beryllium hydride is an amorphous white solid that decomposes above 250 °C giving hydrogen.
It forms its monoxide (BeO) when heated to oxygen. Beryllium oxide is strongly basic in nature and formed by covalent bonding.
The hydroxide, Be(OH)2 can be obtained when treating their monoxide with water or when adding alkali to an aqueous solution of the salts.
Carbides, Nitrides, and Halides of Beryllium
The carbide of Be has the chemical formula Be2C. It is formed by direct reaction with carbon at 1900 °C. It is also obtained by heating metal with acetylene at around 400 °C.
The alkaline earth metal Be burns in nitrogen to form crystalline solid nitrides like Be3N2.
All the metals of group 2 combine with halogen at a suitable temperature to form dihalides like BeCl2 or MgCl2. All the halides of beryllium are covalent and polymeric in nature. Therefore, they do not conduct electricity in the molten phase of the metal.
Organometallic Compounds
Beryllium alkyls can be made by a reaction,
HgMe2 + Be → Hg + BeMe2 at 110 °C
The aryl compounds of the metal are formed during the reaction of lithium aryl (in a hydrocarbon) with BeCl2 in ether. These compounds are viscous liquids or solids that are spontaneously flammable in air and rapidly hydrolyzed by a water molecule.
Uses of Beryllium
Beryllium is used mostly to improve the properties of transition metals like copper and nickel. About two percent of Be increases the strength of Cu six-fold.
The beryllium alloys are non-magnetic, corrosion-resistant, and possess high strength and good electrical conductance. Therefore, they have extensive applications in critical moving parts of aerospace or electrical industries like high-speed aircraft, guided missiles, spacecraft, satellites, and nozzles of liquid-fuel rockets
The metal beryllium is also used in nuclear power plants as a moderator and as a window material of electromagnetic spectrum or x-ray tubes.