Acetaldehyde Molecular Formula
Acetaldehyde (also called ethanal) is a colurless, pungent-smelling organic compound with the molecular formula CH3CHO or C2H4O. It is prepared industrially by the dehydrogenation or air oxidation of ethanol in the presence of a silver catalyst at 300 °C. Acetaldehyde is used in the preparation of several chemical compounds and synthetic resins like acetic acid, ethanol, rubber accelerator, phenolic resins, synthetic drugs, etc. Acetaldehyde occurs naturally in several foods like coffee, bread, and ripe fruit. It is also produced by the partial oxidation of ethanol by the liver enzyme dehydrogenase. The structure and hybridization of the acetaldehyde molecule are given below the picture,
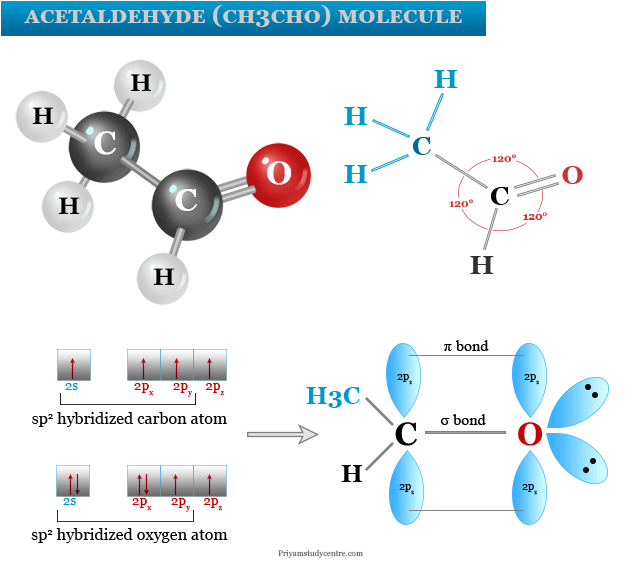
Structure of Acetaldehyde
The molecular formula shows that an aldehyde (-CHO) functional group is bound to a methyl group (-CH3) in an acetaldehyde molecule. Therefore, it is the second simplest aldehyde after formaldehyde.
The chemical structure of acetaldehyde shows that one carbon atom present in the aldehyde functional group is sp2 hybridized while another carbon atom present in the methyl group is sp3 hybridized. Therefore, the CH3CHO molecule has a planar-trigonal together with tetrahedral geometry.
Preparation of Acetaldehyde
- Acetaldehyde can be prepared industrially by the dehydrogenation or air oxidation of alcohol in the presence of a silver catalyst at 300 °C.
CH3CH2OH → CH3CHO - When acetylene passes into dilute sulfuric acid at 60 °C in the presence of a mercuric sulfate catalyst, acetylene adds one molecule of water to form acetaldehyde.
CH ≡ CH + H2O → [CH2 = CHOH] → CH3CHO - It can be prepared by passing a mixture of ethylene and oxygen under pressure into an aqueous solution of palladium and cupric chloride at 50 °C.
CH2 = CH2 + PdCl2 + H2O → CH3CHO + Pd + 2HCl - It can be also prepared by hydroformylation of methanol with chemical catalysts like cobalt, nickel, or iron salts.
Chemical Reactions
Oxidation reactions
Acetaldehyde can easily be oxidized by acid dichromate and permanganate to form acetic acid.
CH3CHO + [O] → CH3COOH
Reduction Reactions
Acetaldehyde is very easily oxidized. Therefore it is a powerful reducing agent. It reduces Fehling’s solution (an alkaline solution containing a complex of copper tartrate) to red curious oxide.
CH3CHO + 2 Cu+2 + 5 OH− → RCOO− + Cu2O + 3 H2O
It also reduces Tollens reagent or ammonical silver nitrate solution to form metallic silver.
CH3CHO + 2 Ag(NH3)2OH → RCO2NH4 + 2 Ag + 3 NH3 + H2O
Condensation Reactions
Acetaldehyde undergoes condensation in the presence of dilute sodium hydroxide, potassium carbonate, or hydrochloric acid to form a syrupy liquid. It is known as aldol.
2 CH3CHO → CH3CH(OH)CH2CHO
On heating, aldol eliminates water to form unsaturated compounds like crotonaldehyde.
CH3CH(OH)CH2CHO → CH3CH = CHCHO + H2O
In Strecker synthesis, CH3CHO condenses with ammonia and hydrogen cyanide to give an amino-nitrile compound. It is further hydrolyzed to give the amino acid alanine.
Polymerization Reactions
When acetaldehyde is treated with a few drops of concentrated sulfuric acid, a vigorous reaction takes place by the formation of a polymer like paraldehyde (CH3CHO)3. It is a pungent-smelling liquid with a boiling point of 128 °C. Paraldehyde is used in medicine as a hypnotic.
When acetaldehyde is treated with a few drops of concentrated sulfuric acid at 0°C, the tetramer metaldehyde (CH3CHO)4 is formed. It is a white solid with a melting point of 246 °C. It regenerates CH3CHO by distilling sulfuric acid.
Uses of Acetaldehyde
Acetaldehyde has a wide range of applications. It is used as a starting material for the production of several compounds like acetic acid, acetic anhydride, ethyl acetate, n-butanol, etc.
- It is a raw material for manufacturing several household products like paint binders, plasticizers, and baby nappies.
- It is also used to manufacture various types of building materials, fire protection paints, lubricants, and explosives.
- In the pharmaceutical industry, paraldehyde formed from the polymerization of acetaldehyde uses as a sleeping medicine.
- It is used for the production of several types of dyes.
- Metaldehyde is formed by polymerization processes used as a fuel.
- It is used for manufacturing several types of disinfectants and perfumes.
- It can be used for the production of plastics and synthetic rubber.
- In the food industry, it is used for the production of preservatives and flavorings agents. It occurs naturally in several fruit and fruit juices.
- A low level of acetaldehyde is found in several foods like milk products, soy products, pickled vegetables, and non-alcoholic beverages.