Erbium Metal
Erbium is a chemical element or rare earth metal in the periodic table with the symbol Er and atomic number 68. It is also alloyed with vanadium to lower its hardness and improve its workability. Erbium is a soft, silvery metallic element that is used for making optical fiber glass to amplify optical communication signals and laser treatment. Erbium was discovered in 1843 by Swedish chemist Carl Gustav Mosander from the mineral Ytterby in a gadolinite mine found in Sweden. It was one of three elements found in Ytterby that separated from the mineral gadolinite. He discovered the erbium oxide form of the metal and named it erbia after the village of Ytterby.
Where is Erbium Found?
Erbium is never found as a free element in nature but an extractable amount of oxide is found in rare-earth minerals monazite and bastnaesite. It is one of the more abundant rare-earth metals that is mined mostly in China and the US.
It can be separated or extracted by ion exchange chromatography and solvent extraction process.
Isotopes
Naturally occurring erbium has six stable isotopes with atomic masses 162, 164, 166, 167, 168, and 170. It also contains 29 synthetic radioactive isotopes that can be synthesized by various artificial nuclear reactions. Most of these radioactive isotopes have half-lives of less than 4 minutes.
The primary radioactive decay mode of these radioactive isotopes is electron capture or beta decay.
Properties
The element erbium is a silvery-white, malleable rare earth metal belonging to the lanthanide series in the periodic table that is used commonly in optical fiber glass and laser treatment. Like other lanthanides, it commonly shows a +3 oxidation number or state.
| Erbium | |||
| Symbol | Er | ||
| Discovery | Carl Gustav Mosander in 1843 | ||
| Name derived from | It is named after the mineral Ytterby found in Sweden | ||
| Common isotope | 68Er166 | ||
| Oxidation state | +3 | ||
| CAS number | 7440-52-0 | ||
| Periodic properties | |||
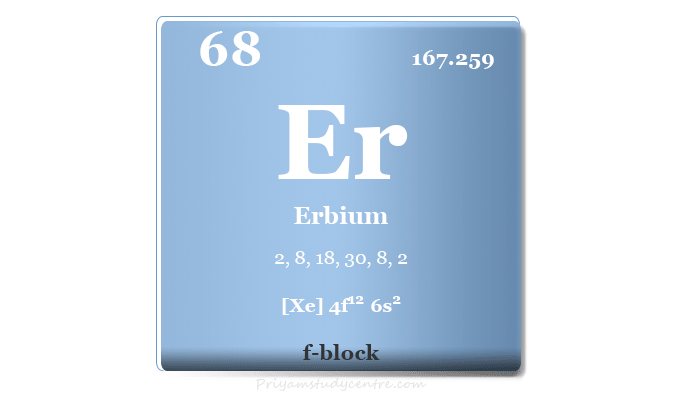
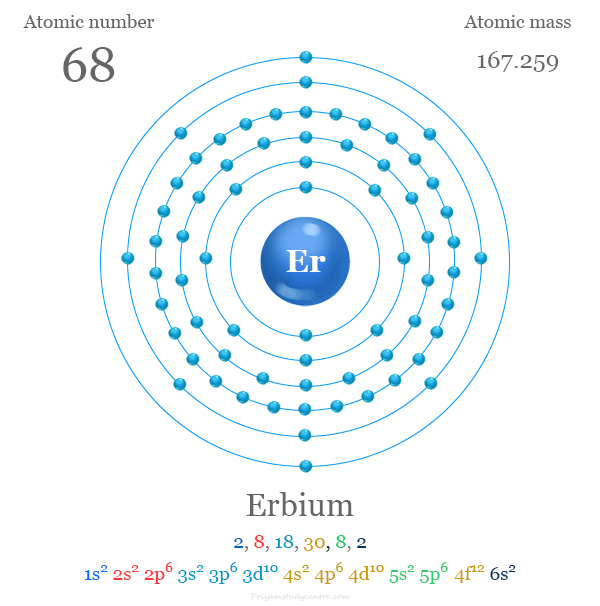
| Atomic number | 68 | ||
| Relative atomic mass | 167.259 | ||
| Electron per cell | 2, 8, 18, 30, 8, 2 | ||
| Electronic configuration | [Xe] 4f12 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1529 °C, 1802 K | ||
| Boiling point | 2868 °C, 3141 K | ||
| Molar heat capacity | 28.12 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 9.07 g/cm3 | ||
| Heat of fusion | 19.90 kJ mol−1 | ||
| Heat of vaporization | 280 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.29 Å | ||
| Covalent radius | 1.77 Å | ||
| Electronegativity | 1.24 (Pauling scale) | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 589.30 | 1151.07 | 2194.08 | |
Electron Configuration for Erbium
The 68 electrons of the erbium atom are distributed in different energy levels to show the following electronic configuration,
Erbium in the Periodic Table
The rare earth metal erbium is placed in the f-block of the periodic table. It is lanthanide that lies between holmium and thulium.
Chemical Properties
It is a reactive metal that is very stable in dry air at ordinary temperatures but slowly dull in humid atmospheres. Erbium burns in the air or oxygen to form oxide Er2O3.
4 Er + 3 O2 → 2 Er2O3
The rare earth metal Er stands far above hydrogen in the electrochemical series. Therefore, it reacts slowly with cold water and rapidly with hot water by the liberation of hydrogen and the formation of metal hydroxide.
2 Er + 6 H2O → 2 Er(OH)3 + 3 H2
Reactions with acids are more vigorous but the Er does not dissolve in alkalies. It dissolves readily in dilute sulfuric acid to form solutions containing the yellow Er (III) ions.
2 Er + 3 H2SO4 → 2 Er3+ + 3 SO4−2 + 3 H2
It is normally from trihalides with halogens like fluorine, chlorine, bromine, and iodine.
2 Er + 3 X2 (X = F, Cl, Br, I) → 2 ErX3
Facts About Erbium
- It is never found in nature as a free element but oxide form is found with the minerals of other rare-earth elements.
- The pure metal was isolated after the discovery of the ion exchange chromatography technique.
- The approximate price or cost of erbium is $650 per kilogram. After the discovery of advances in ion-exchange chromatography techniques, the price has gone down.
- The salts of Er are rose-colored compounds with sharp adsorption spectra in visible, ultraviolet, and infrared light.
- It does not show any biological role in humans but Er salts can stimulate metabolism in our bodies.
- Erbium dope optical fibers and laser glass can amplify the signals in communication technology.
- Pure metal is slightly toxic while compounds tend to be non-toxic in nature.
What is Erbium Used for?
- It is alloyed with vanadium to lower its hardness and improve its workability.
- In communication technology, erbium-doped silica glasses are used for making optical fiber.
- The pink color erbium oxide is used for the coloration of glasses, cubic zirconia, and porcelain.
- It is used in laser surgery treatment due to the unique wavelength of erbium ions.
- Salt of Er increases the red blood corpuscle and hemoglobin content in the blood.
- In nuclear technology, erbium is used for neutron-absorbing material in control rods of nuclear reactors.