Praseodymium Element
Praseodymium is a chemical element or rare earth metal in the periodic table with the symbol Pr and atomic number 59. It is a soft, ductile, and silvery-white metal that lies with f-block elements or lanthanides. The lighter rare earth metal praseodymium was discovered by Austrian chemist Baron Carl Auer von Welsbach in 1885 and the name of the metal was derived from the Greek word prasios didymos meaning leek-green twin. Praseodymium element gives high-power magnets when combined with neodymium or other rare-earth metals or 3d transition metals. These magnets are used widely in motors, printers, watches, headphones, loudspeakers, and magnetic storage.

Naturally, praseodymium is found together with other rare-earth metals. The electronic configuration of Pr is [Xe] 4f3 6s2. Like other lanthanides, it shows a +3 oxidation number or state in an aqueous solution. The +4 oxidation state of praseodymium is also found in many solid compounds.
Where is Praseodymium Found?
Naturally, praseodymium is found in the Earth’s crust with an abundance of 9.5 parts per million. It also occurs in seawater with an abundance of 1 part per trillion.
Praseodymium is found commercially in two main ores such as monazite and bastnasite. Monazite is chemically quite inert and mostly concentrated in beach sands and river beds through weathering.
Monazite is found mainly in China, USA, Brazil, India, South Africa, Sri Lanka, Australia, and Malaysia. Bastnasite occurs mainly in USA and China.
Isotopes
The lanthanide praseodymium has only one naturally occurring isotope like 141Pr. 38 other radioactive isotopes may be synthesized by various nuclear reactions. The radioactive isotopes lighter than 141Pr are converted to cerium isotopes by positron emission or electron capture reactions.
All of these radioactive isotopes have very short half-lives (under a day). The isotopes 143Pr and 141Pr may be found during the fission of uranium in nuclear reactors. The isotope, 147Pr is used for the production of atomic batteries.
Properties
It is a soft silver-colored rare earth metal that develops a green oxide coating in the air. The softness of praseodymium compared to that of silver.
The third member of the lanthanide series, praseodymium adopts a double hexagonal close-packed crystal structure at room temperature.
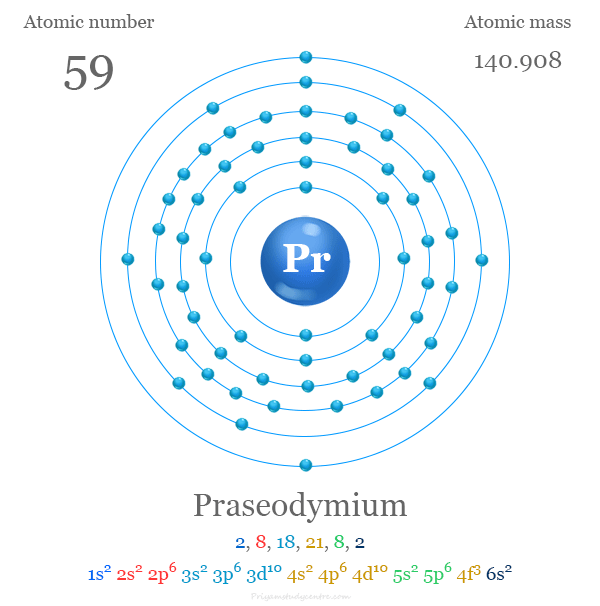
The electron configuration of metal Pr is [Xe] 4f3 6s2. Therefore, it has five valence electrons for forming chemical compounds but the use of all five requires extreme conditions. Normally, praseodymium uses three or sometimes four electrons for chemical bonding with other atoms.
| Praseodymium | |||
| Symbol | Pr | ||
| Discovery | Carl Auer von Welsbach in 1885 | ||
| Name derived from | Greek word prasios didymos meaning green twin | ||
| Common isotope | 59Pr141 | ||
| Oxidation states | +4, +3 | ||
| CAS number | 7440-10-0 | ||
| Periodic properties | |||
| Atomic number | 59 | ||
| Relative atomic mass | 140.908 | ||
| Electron per cell | 2, 8, 18, 21, 8, 2 | ||
| Electronic Configuration | [Xe] 4f3 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 931° C, 1204 K | ||
| Boiling point | 3520 °C, 3793 K | ||
| Molar heat capacity | 27.20 J mol−1 K−1 | ||
| Crystal structure | double hexagonal close-packed (dhcp) | ||
| Density | 6.77 g/cm3 | ||
| Heat of fusion | 6.89 kJ mol−1 | ||
| Heat of vaporization | 331 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.40 Å | ||
| Covalent radius | 1.90 Å | ||
| Electronegativity | 1.13 (Pauling scale) | ||
| Electron affinity | 92.814 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 528.06 | 117.92 | 2086.40 | |
Praseodymium in the Periodic Table
It is found with f-block elements in the periodic table and third member of the lanthanide series. The rare earth metal praseodymium lies between cerium and neodymium.
Facts About Praseodymium
- In 1841, Pr was identified by Swedish chemist Carl Mosanderin in rare-earth oxide residue didymium but he did not purify it. Pr was discovered by Austrian chemist Baron Carl Auer von Welsbach in 1885.
- The active metal Pr is highly malleable and ductile.
- Like other lanthanides, praseodymium principally shows a +3 oxidation state or number. In addition to the +3 oxidation state, the +4 oxidation state of Pr is also found in many solid compounds.
- It forms a green oxide coating in the air. Therefore, to prevent degradation, pure praseodymium is stored under a protective atmosphere or in oil.
- It is alloyed with other metals to make stable and powerful rare earth magnets that are used in small equipment.
- It contains only one stable naturally occurring isotope, Pr-141.
- Like other rare earth metals, praseodymium may not serve any known biological function.
- Pr is not very toxic but many side effects appear during inhalation due to radioactive thorium and uranium impurities.
Chemical Properties
In general lanthanide, praseodymium behaves like an active metal. The electrode potential value of Pr is comparable to that of alkali metals. It acts as a strong reducing agent.
Praseodymium metal tarnishes slowly in the air by the formation of a green oxide layer. Pr burns readily in oxygen at 150 °C to form a nonstoichiometric praseodymium oxide with the chemical formula Pr6O11.
12 Pr + 11 O2 → 2 Pr6O11
It is attacked by acids like dilute sulfuric acid with the liberation of hydrogen gas. The acidic solutions contain Pr3+ ions that exist as [Pr(H2O)9]3+ complexes.
2 Pr + 3 H2SO4 → 2 Pr3+ + 3 SO4−2 + 3 H2
The metal praseodymium dissolves slowly in cold water but more rapidly in warm water with the liberation of hydrogen. It forms Pr (III) hydroxide with water.
2 Pr + 6 H2O → 2 Pr(OH)3 + 3 H2
Praseodymium metal reacts with all the halogens like chlorine, fluorine, bromine, or iodine to form trihalides. PrF3 is precipitated by the addition of HF or a soluble fluoride to a Pr+3 salt solution.
The anhydrous chloride can be prepared by the direct combination of the elements on heating. It may also be prepared by heating the praseodymium oxide with carbonyl chloride or ammonium chloride.
Pr2O3 + 3 COCl2 → 2 PrCl3 + 3 CO2
Pr2O3 + 6 NH4Cl → 2 PrCl3 + 3 H2O + 6 NH3
Praseodymium carbonate can be prepared by passing carbon dioxide into an aqueous solution of Pr(OH)3. It can also be prepared by adding sodium carbonate solution to the Pr+3 salt solutions.
Uses of Praseodymium
- The oxide form praseodymium is used for glass coloration. It is used for making yellow-green glasses named Prasemit.
- It also gives yellow color enamels and ceramics.
- The mixture of Nd2O3 and Pr2O3 is used for making goggles that we use for glass blowing and welding work.
- Praseodymium gives high-power magnets when combined with neodymium or another rare-earth element.
- The combination of praseodymium and 3d-transition metals give extremely stable magnets that we used in motors, printers, watches, headphones, loudspeakers, and magnetic storage.
- Mischmetal contains about 5% praseodymium. It is used to make flints for cigarette lighters.
- It alloys with magnesium to create high-strength alloys that are used in aircraft engines.
- The isotope of praseodymium, 147Pr is used in the production of atomic batteries.