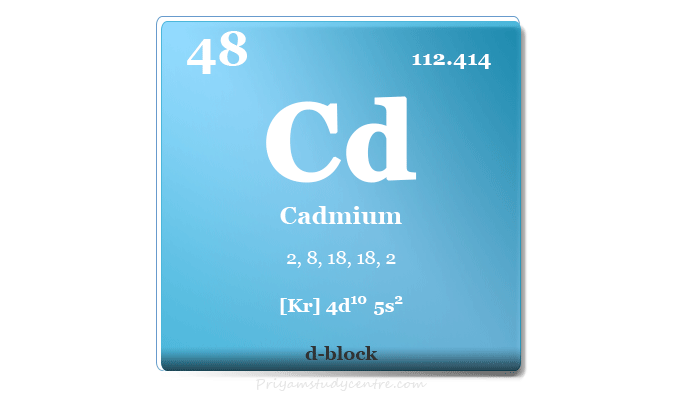
Cadmium Element
Cadmium is a chemical element of group 12 metal in the periodic table with the symbol Cd and atomic number 48. It is a silvery white soft metal chemically similar to that of group member zinc and mercury. It is used widely in cadmium plating of steel to prevent corrosion and rechargeable batteries made by nickel-cadmium cells. The use of cadmium generally decreases due to its toxic nature. The elements Zn, Cd, and Hg are the last members in each d-series but have no characteristics of transition metals. These elements contain filled d-orbital and do not show variable oxidation numbers or states, colour arising out of d-d transition, and paramagnetic complexes. Cadmium was discovered in 1817 by German chemists Stromeyer and Hermann from an impurity in zinc carbonate. The name of the metal is derived from the Latin word cadmia which is obtained from the mineral name calamine.
Where is cadmium found?
A small quantity of Cd is found in most soil and rocks, coal, and mineral fertilizers. Cadmium minerals are usually combined with other elements such as oxygen, chlorine, or sulfur.
Cadmium is mostly found in association with zinc but it also occurs rarely in the sulfide mineral greenockite (CdS). A high level of world-produced cadmium is obtained from China, South Korea, Japan, and North America. Most of the commercially produced cadmium is obtained as a by-product during zinc refining.
The carcinogenic element cadmium can still be found in everyday household products like second-hand plastic toys, drinking glasses, alcoholic beverage bottles, ceramics, and various paints.
Production process
Cadmium invariably occurs as an impurity in zinc and the metal is recovered as a by-product of zinc production.
- The two elements have reasonable differences in boiling point. Therefore, Cd can be separated from Zn by fractional distillation.
- Zinc is more electropositive than cadmium. Hence cadmium may also be precipitated from a Cd (II) solution by metallic zinc.
Cd+2 + Zn → Cd + Zn+2 - The standard potentials of Zn and Cd are different. Therefore, during the electrolysis of a Cd-reached zinc sulfate solution may be deposited pure Cd on the cathode before any Zn deposit.
Isotopes
Cadmium has eight naturally occurring isotopes with atomic masses 106, 108, 110, 111, 112, 113, 114, and 116. Among these, the three isotopes 110Cd, 111Cd, and 112Cd are stable but 113Cd and 116Cd are radioactive. The remaining three naturally occurring isotopes (106Cd, 108Cd, and 114Cd) are expected to radioactive decay but have not been performed under laboratory conditions.
Many other radioactive isotopes of Cd have been produced by various artificial nuclear reactions. Of these radioactive isotopes, the most stable are 109Cd and 115Cd. The half-lives of the majority of these radioactive isotopes have less than 5 minutes. The primary decay mode of Cd isotopes is electron capture to produce silver and beta emission to produce indium.
Properties
Cadmium is a lustrous silvery metal with a bluish tinge and tarnishable in moist air. It forms a distorted hexagonal closed-packed metallic crystal lattice with an elongated distance between closed-packed layers.
The density of Cd is slightly lower than those of copper and silver. It is a softer and low-melting metal in comparison to other transition metals.
| Cadmium | |||
| Symbol | Cd | ||
| Discovery | Friedrich Stromeyer in 1817 | ||
| Name derived from | The Latin word cadmia is obtained from the mineral name calamine | ||
| Common isotope | 48Cd114 | ||
| Oxidation states | 2 | ||
| CAS number | 7440-43-9 | ||
| Periodic properties | |||
| Atomic number | 48 | ||
| Relative atomic mass | 112.414 | ||
| Electron per cell | 2, 8, 18, 18, 2 | ||
| Electronic configuration | [Kr] 4d10 5s2 | ||
| Block | d-block | ||
| Group | 12 | ||
| Period | 5 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 321.069 °C, 594.219 K | ||
| Boiling point | 767 °C, 1040 K | ||
| Molar heat capacity | 26.020 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 8.69 g/cm3 | ||
| Electrical resistivity | 72.7 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.18 Å | ||
| Covalent radius | 1.40 Å | ||
| Electronegativity | 1.69 (Pauling scale) | ||
| Electron affinity | unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 867.78 | 1631.40 | 3616.27 | |
Electron configuration
The 48 electrons of the Cd atom are distributed in different energy levels to show the following electron configuration given below the picture,
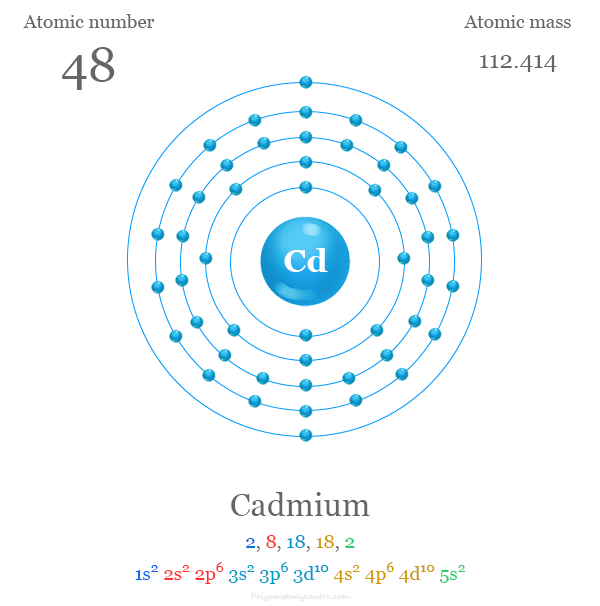
The metals zinc, cadmium, and mercury contain ns2 outer electron configurations like alkaline earth metals calcium, strontium, and barium. But the first and second ionization energies of these d-block elements are considerably higher than those of calcium, strontium, and barium.
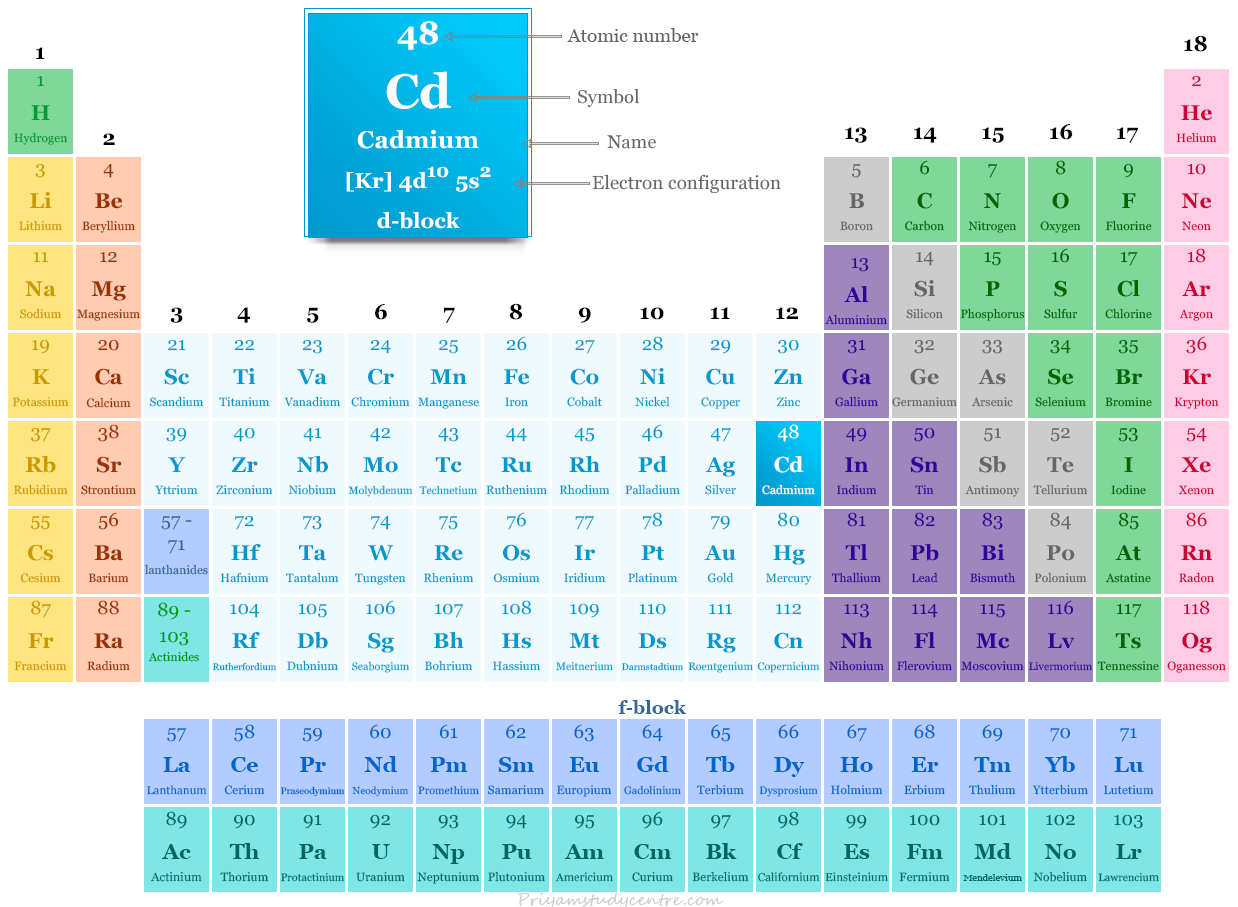
Cadmium in the periodic table
Cadmium is placed in group 12 and period 5 in the periodic table. It is a d-block element that lies between silver and indium.
Chemical properties
The only stable oxidation state for Cd is +2. Like mercury, it also shows a +1 oxidation state. Oxide, sulfide, and halides are the most important compounds of cadmium but hydride, nitride, and halides are unstable. Many complexes of cadmium with nucleobases, amino acids, vitamins, and enzymes have been also determined.
It burns in the air to brown amorphous cadmium oxide (CdO). The dark red crystalline solid CdO is obtained when heating. It adopts a sodium chloride-type crystal lattice. The dark red colour also changes to black when heating due to lattice defects.
Cadmium may dissolve in mineral acids like hydrochloric acid, sulfuric acid, or nitric acid by liberation of hydrogen and formation of CdCl2, (CdSO4), or Cd(NO3)2.
The addition of sodium hydroxide or potassium hydroxide to the Cd+2 solution precipitates white Cd(OH)2. It may be dissolved in concentrated alkali on prolonged boiling to form hydroxocadmiates, K4[Cd(OH)6].
The large difference between the 1st and 2nd ionization energies for Cd suggests the formation of a Cd+ ion. In practice, like the mercury (I) state, the univalent state appears in the form of Cd2+2. It is readily disproportionate in an aqueous solution to form Cd and Cd+2 ions.
Cd2+2 → Cd↓ + Cd+2
The sulfide of Cd is very familiar to us in the routine group analysis of Cd+2. CdS precipitated by hydrogen sulfide from a dilute HCl medium because CdS requires a faintly acid medium for complete precipitation.
Facts about cadmium
- The soft, malleable, ductile, and silvery-white cadmium is insoluble in water.
- The bulk form of metal is not flammable but the powdered form may burn to release toxic fumes.
- Cadmium mainly occurs in combination with zinc but is extracted as a by-product in zinc, lead, and copper refining
- Naturally, a very large amount of Cd is released into our environment through weathering of rocks, forest fires, and volcanoes. A significant amount of Cd also is released through various human activities.
- The elements Zn, Cd, and Hg belong to the d-block but are not included among the transition series of elements as they do not form any compounds in which the d-shell is partially occupied.
- However, the elements (Zn, Cd, and Hg) resemble transition metals in forming complex compounds with a variety of ligands like ammonia, amines, halide ions, and cyanide. But the complexes with other strong π-acceptor ligands are not known.
- Most of the cadmium produced today from zinc byproducts is spent to make nickel-cadmium batteries.
- Helium–cadmium lasers are a common source of blue or ultraviolet laser light at wavelengths of 325, 354, and 442 nm. These lasers are used in fluorescence microscopy and various laboratory equipment that require laser light at these wavelengths.
Uses of cadmium
The use of cadmium decreased in recent years due to its toxic behavior. It causes birth defects and cancer. It is used mainly to make batteries, alloys, coatings (electroplating), solar cells, plastic stabilizers, and pigments.
- Most of the cadmium produced today is used for the production of rechargeable batteries made by nickel-cadmium cells. These batteries have been replaced today with nickel-metal hydride and lithium-ion batteries due to the toxicity of Cd.
- It is used largely in cadmium plating of steel to prevent corrosion. These alloys of Cd are still used to protect critical components of airplanes and oil platforms.
- It is a component of some semiconductor materials.
- Cadmium sulfide, selenide, or telluride is used in some photodetectors and solar cells for the production of solar energy.
- Heavy metal complexes give great advantages for the treatment of cancers but their use is often limited due to the toxic side effects of cadmium.
- Historically, cadmium compounds may be used as phosphors in black-white TV sets, colour TV sets, and yellow, orange, and red pigments.
- Cadmium compounds are used for the stabilization of PVC against degradation by heat and ultraviolet-visible radiation. Currently, Cd stabilizers have been completely replaced with barium-zinc, calcium-zinc, and organo-tin stabilizers.
- Cadmium rods are used in nuclear power reactors as an absorber of neutrons to control nuclear fission.
- Cadmium amalgam is used in laboratories for reducing organic compounds.
Cadmium toxicity
Cadmium enters the water through industrial discharges or the deterioration of galvanized pipes. It is toxic to certain fish at concentrations above 200 ppb while the normal level of Cd in portable water is 0.4 to 60 ppb.
It may also concentrate in plants where they enter with zinc. The small amount of cadmium that enters the human body has been eliminated through the kidneys but the residual fraction is bound by body protein. It ultimately leads to kidney disinfection.
Excessive daily dose or inhalation may cause the following disorders in human health,
- Diarrhea, stomach pains, and vomiting
- Reproductive failure
- Bone fracture
- Damage to the immune system and the central nervous system
- Psychological disorders
- DNA damage or cancer development
Cadmium may replace zinc in our body and inhibit the action of zinc enzymes.








