Rhodium Metal
Rhodium metal is one of the rarest and precious metals found in group 9 of the periodic table with the symbol Rh and atomic number 45. It is a silvery-white, hard, corrosion-resistant transition metal that is found in platinum or nickel ores. The metal rhodium is expensive or high price and is used for electroplating gold, silver, and platinum jewelry to improve their appearance. The metal palladium and rhodium were discovered by English physicist and chemist Sir William Hyde Wollaston in the same year (1803). After this discovery, palladium is considered a daily demanding metal in the world market but rhodium is less well known. It is used in the catalytic converter of every new car. Therefore, the value of rhodium very much depends on the demand from the automotive industry.

At this time it was not used widely due to the rarity of abundance, difficulty in shaping, and high melting point. However, as the years passed, it was used for electroplating and corrosion-resistant coating in jewelry. The catalytic converter was discovered in 1976. After this discovery, the demand and price of rhodium metal increased substantially. The price of the metal can also be affected by the level of supply from the main exporter South Africa followed by Russia.
Where is Rhodium Found?
Rhodium is one of the rarest metals in the Earth’s crust found in platinum ores and extracted as white inert metal. Rhodium is found with other platinum metals in river sands of the Ural Mountains in Russia, South Africa, and North and South America. A very small concentration of metal is found in the copper-nickel sulfide mining area of the Sudbury, Ontario, region.
The estimated abundance of the metal in the earth’s crust is about 0.0002 parts per million. The rarity of abundance affects the commercial applications and price of rhodium. The main exporter of rhodium is South Africa followed by Russia and the annual world production of the metal is about 30 tonnes.
Isotopes
Rhodium metal has only one naturally occurring stable isotope with atomic mass 103. Many other radioactive isotopes have been produced by various artificial nuclear reactions. Of these radioactive isotopes, the most stable are 101Rh, 102Rh, 102mRh, and 99Rh. The half-lives of most of these radioactive isotopes are shorter than an hour.
The primary radioactive decay mode of Rh isotopes is electron capture and beta emission. The primary decay products of radioisotopes are ruthenium and palladium.
Rhodium is obtained during the nuclear fission of uranium-235. A significant amount of the lighter platinum group metals are obtained during the nuclear fission of uranium. Therefore, nuclear fuel is a potential source of Rh metal.
Properties
Rhodium is a silvery-white, hard, and very shiny metal that reflects up to 80 percent of light.
| Rhodium | |||
| Symbol | Rh | ||
| Discovery | William Hyde Wollaston in 1803 | ||
| Name derived from | The Greek word rhodon means rose coloured | ||
| Common isotope | 45Rh103 | ||
| Oxidation states | +5, +4, +3, +2, +1, 0 | ||
| CAS number | 7440-16-6 | ||
| Periodic properties | |||
| Atomic number | 45 | ||
| Relative atomic mass | 102.906 | ||
| Electron per cell | 2, 8, 18, 16, 1 | ||
| Electronic configuration | [Kr] 4d8 5s1 | ||
| Block | d-block | ||
| Group | 9 | ||
| Period | 5 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1963 °C, 2236 K | ||
| Boiling point | 3695 °C, 3968 K | ||
| Molar heat capacity | 16.443 J mol−1 K−1 | ||
| Crystal structure | face-centered cubic (fcc) | ||
| Density | 1.85 g/cm3 | ||
| Electrical resistivity | 43.3 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.10 Å | ||
| Covalent radius | 1.34 Å | ||
| Electronegativity | 2.28 (Pauling scale) | ||
| Electron affinity | 109.704 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 719.67 | 1744.45 | 2996.83 | |
Electron Configuration
The 45 electrons of the rhodium atom are distributed in different energy levels to show the following electron configuration given below the picture,
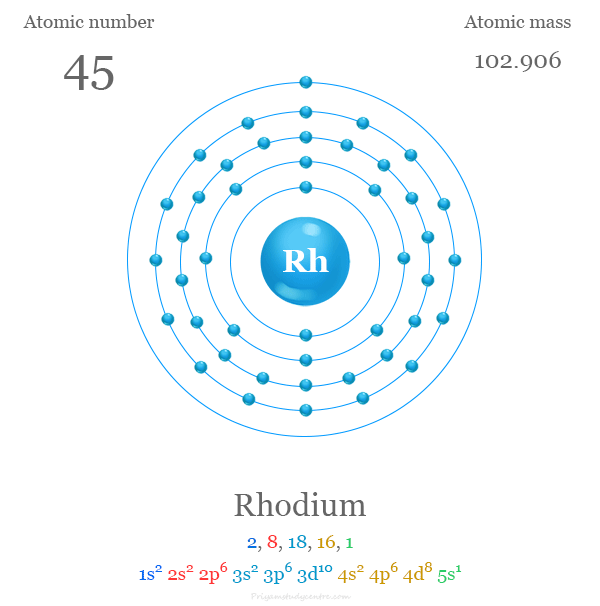
Rhodium in the Periodic Table
The platinum metal rhodium is placed in group 9 and period 5 in the periodic table. It is a d-block element or transition metal that lies between ruthenium and palladium.
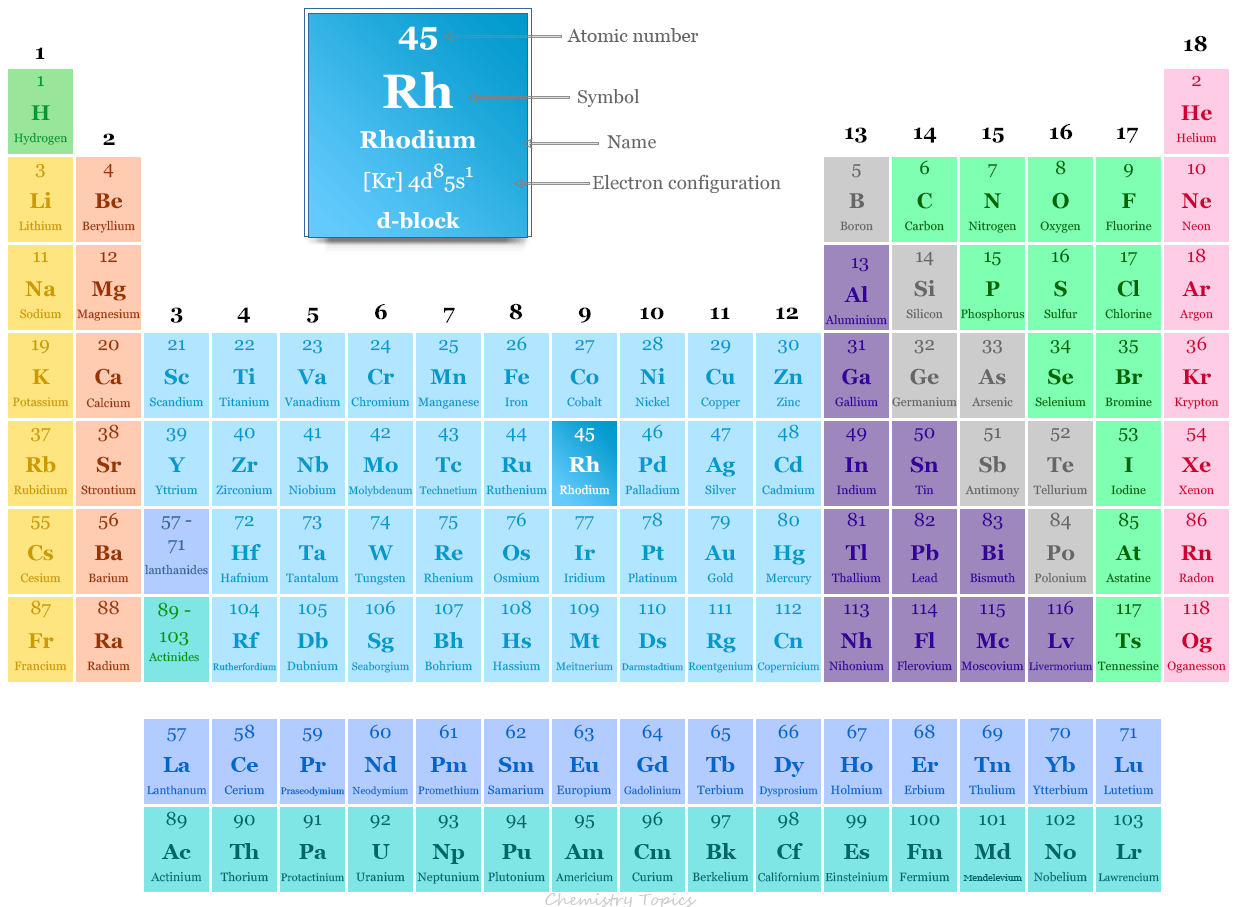
Production Process
Industrial extraction is complex because rhodium-bearing ores are mixed with other metals such as palladium, silver, platinum, and gold. It is found in platinum ores and extracted as a white inert rhodium metal.
The refining of rhodium from other platinum metals is given below,
- The solution of rhodium (III) sulfate obtained from anode slime of platinum metals precipitates Rh(OH)3 with aqueous sodium hydroxide.
- The precipitate dissolved in hydrochloric acid to give H3[RhCl6].
- Addition of NaNO2 and then NH4Cl in this solution produced (NH4)3[Rh(NO2)6].
- When this complex is digested in HCl, the soluble chloro complex is formed.
- The solution is evaporated and ignited in hydrogen to produce rhodium metal.
Chemical Compounds
In addition to the noble character and catalytic behavior, platinum metals have several common features in their chemistry. All of them form a wide variety of binary compounds like oxides, sulfides, and especially halides.
As compared with the previous pair of heavy transition elements, Rh and Ir have a lower tendency to attain higher oxidation states. The highest state found is VI and the most stable state for Rh is III.
Rhodium Oxides
Rhodium has only a few compounds in oxidation state IV. On heating air, Rh forms Rh2O3. The oxide RhO2 can be made by strong oxidation of Rh (III) in an alkaline solution and dehydrating under high pressure.
In oxidation state III, the element forms oxide, halide, and many complex compounds. Dark gray Rh2O3 is formed by heating Rh metal in oxygen at 600 °C. Rhodium (III) oxide has a corundum structure.
Rhodium Halides
Except iodide Rh trihalides may be prepared by direct reaction of metal with halogens like chlorine, fluorine, and bromine. RhI3 is obtained by adding aqueous KI to the tribromide.
They are red or red-brown solid except RhI3 is black. The anhydrous trihalides are usually insoluble in water but water-soluble hydrates may be prepared by dissolving the hydrous oxide in appropriate HX (X = F, Cl, Br, I).
The red-brown RhF6 is an unstable compound that oxidizes water vigorously. In the absence of water, RhF6 can oxidize chlorine molecules and NO.
2 RhF6 +3 Cl2 → 2 RhF3 + 6 ClF
NO + RhF6 → NO+[RhF6]−
Complex Compounds
Rh2O3, xH2O dissolve in acids to form yellow Rh(H2O)6+3 ion. It forms alums that are structurally similar to those of Co(H2O)6+3 but more stable to reduction.
Generally, the complexes of Rh (III) are octahedral. The octahedral complexes are formed by ligands like water and ammonia.
The oxidation state II is not common for Rh and no established oxide or halide is known in this state. Complexes like [Rh(dipy)2Cl]+ and [Ph3PRh(OCOCH3)2]2 are known. The dimeric acetate of Rh (II) is obtained by heating RhCl3.3H2O with a methanolic solution of sodium acetate.
Interesting Facts About Rhodium
- The third platinum metal rhodium was discovered from crude platinum in the same year (1803) when palladium was discovered.
- The name of the metal comes from the rose red colour of the solution of Rh salts. The name is derived from the Greek word rhodon meaning rose.
- It may not play any biological role in humans and other animals.
Uses of Rhodium
The uses of rhodium metal significantly influence by its rarity in appearance and high price. The most common uses of this metal are given below,
Automobile Industry
Rhodium metal primarily uses in the automobile industry as a catalytic converter for changing harmful unburned hydrocarbons, carbon monoxide, and nitrogen oxide.
Today, the majority of car manufacturers use this metal as a catalytic converter to minimize pollution. Therefore, the price of rhodium metal very much depends on the demand from the automotive industry.
Chemical Catalyst
It is a useful chemical catalyst for making nitric acid from ammonia and acetic acid from methanol. Rhodium catalyst is used to catalyze hydrosilanes to manufacture certain silicone rubbers. The catalyst is also used to reduce benzene to cyclohexane.
The phosphine complexes of metal are used as a catalyst in hydrogenation and hydroformylation reactions. It is preferable to other platinum metals for the reduction of nitrogen oxides to nitrogen and oxygen.
Glass Industry
It is also the most common use of metal. Rhodium is used for making flat-panel glass and fiberglass.
Rhodium for Jewelry
The rarer metal rhodium is the whitest and most precious metal used for jewelry. Rhodium plated on white gold and platinum gives a reflective white surface at a time to sell the jewelry. When coated with a thin layer, it gives extra strength and luster.
It may also be used for coating silver to protect against tarnish or protect silver from atmospheric hydrogen sulfide.
Rhodium-plated jewelry provides an extra layer of protection to jewelry. It also produces a brighter white than sterling silver, white gold, or platinum. Rhodium plating does not scratch, dent, or corrode the jewelry which we want to sell.
Alloying Platinum and Palladium
It is used for alloying platinum and palladium to improve hardness and corrosion resistance. These alloys are used in furnace windings, bushings for glass fiber, turbine reactors, electrodes for aircraft spark plugs, electric ovens, laboratory crucibles, etc.
Other Uses
- It is used to coat optical fibers and optical mirrors.
- It is also used for coating crucibles, thermocouple elements, and headlight reflectors.
- It is used to make electrical contacts because it has small and stable electrical resistance. But commonly, we use ruthenium instead of rhodium due to the reality of appearance and the high price of Rh metal.
- Rhodium neutron detectors are used in nuclear power reactors to measure neutron flux levels.
Price of Rhodium
Rhodium is used in the catalytic converter of every new car produced today. Therefore, the value of rhodium very much depends on the demand from the automotive industry. The price of the metal can also be affected by the level of supply from the main exporter South Africa and Russia.
The price of rhodium highly varies with time. In 2007, the price of rhodium was eight times more than gold, 450 times more than silver, and 27,250 times more than copper. In 2008, the price rose to go above $10,000 per ounce or $350 per gram.
The price of rhodium was less than $1,000 per ounce in late 2013 due to various Political and financial problems or small demand for metal in the automotive industry.
The price of rhodium metal decreased below $8,000 per ounce in May 2020. In April 2021, the price rose to a new high level of $ 28,775 before decreasing towards the end of 2021. In August 2022, the average price of rhodium metal decreased again reaching $14,000 per troy ounce.








