Ruthenium Element
Ruthenium is a group 8 chemical element or metal in the periodic table with the symbol Ru and atomic number 44. The demand and price for ruthenium are rising due to the widespread use of metal in the electronic and chemical industries as a catalyst. In these two industries, ruthenium metal is used mainly for making electrical contacts and anode plates for chlorine production in electrochemical cells. Ruthenium metal is the rarest transition metal on the earth that imparts hardness to platinum jewelry and palladium. It is also used in solar technology for the production of electrical energy from solar energy.
Ruthenium is a hard white metal that is a member of the platinum group metals (PGMs). Ruthenium was discovered in 1844 by Russian scientist of Baltic-German ancestry Karl Ernst Claus. The name of the metal was given in honor of the country Russia.
Where is Ruthenium Found?
Ruthenium is one of the rarest metals on Earth and is generally found in ores of other platinum metals in the Ural Mountains and in North and South America. The chief source of the metal is found in South Africa, Canada, Russia, Brazil, Columbia, etc.
It is the 78th most abundant element in Earth’s crust that is found in the uncombined form in nature. Commercially, it can be extracted from the wastes of nickel refining.
The abundance of platinum metals in the earth’s crust is 0.01 ppm or less. Ruthenium is relatively rare on Earth and found in about 100 parts per trillion. Mostly, the element occurs in the minerals pentlandite and pyroxinite.
Ruthenium Isotopes
Ruthenium metal has seven naturally occurring stable isotopes. It has also 27 radioactive isotopes that have been produced by various artificial nuclear reactions. Of these 27 radioactive isotopes, the most stable are 106Ru, 103Ru, and 97Ru. The half-lives of most of these radioactive isotopes have less than five minutes.
The primary radioactive decay mode of these isotopes is electron capture and beta emission. The primary decay products of radioisotopes are technetium and rhodium.
The concentration of ruthenium is relatively high during the fission of nuclear power reactors. Therefore, most long-lived Ru-106 may also be extracted from radioactive waste. The isotope Ru-106 is found during the nuclear fission of uranium or plutonium.
Production Process
About 30 tonnes of ruthenium are mined each year in the World. The composition of the mined platinum group metals varies widely on the geolocation. For example, the platinum group metals mined in South Africa contain on average 11% ruthenium while the platinum group metals mined in the former USSR contain only 2%.
Ruthenium is obtained commercially as a by-product from nickel, copper, and platinum metals ore refining. During the electrorefining of copper and nickel, noble metals such as silver, gold, and the platinum group metals precipitate as anode sludge.
Ruthenium Refining
The refining of ruthenium and other platinum metals from the anode sludge is given below,
- Treatment of the anode slime with aqua regia removes platinum, palladium, and gold. It may form soluble salts of PtCl6−2, PdCl4−2, and AuCl4−2.
- The residue of insoluble chlorides of osmium, ruthenium, rhodium, iridium, and silver is fused with lead carbonate followed by treatment with nitric acid. Silver is passed into the solution as silver nitrate.
- After the removal of silver, the insoluble residue is fused with NaHSO4 and leached with water. Rhodium passes into solution as Rh2(SO4)3.
- The insoluble residue after separating rhodium is fused with sodium peroxide and leached with water. Ru and Os pass into the solution as RuO4−2 and [OsO4(OH)2]−2 but iridium is left as a residue of IrO2.
- Chlorine is passed into the solution containing Ru and Os. On heating the solution, OsO4 vaporized and separated.
- After removing osmium, the solution contains H3RuCl6. From which (NH4)3RuCl6 is precipitated by NH4Cl. The precipitate is heated with hydrogen to give ruthenium metal.
Properties
Ruthenium is a hard, brittle, silvery-white transition metal that does not react normally with acids, water, or air. It belongs to the noble metals or platinum metals family in the periodic table.
| Ruthenium | |||
| Symbol | Ru | ||
| Discovery | Karl Karlovich Klaus in 1844 | ||
| Name derived from | The word Ruthenia that has the Latin name for Russia | ||
| Common isotopes | 44Ru101, 44Ru102, 44Ru104 | ||
| Oxidation states | +8, +6, +4, +3, +2, 0, −2 | ||
| CAS number | 7440-18-8 | ||
| Periodic properties | |||
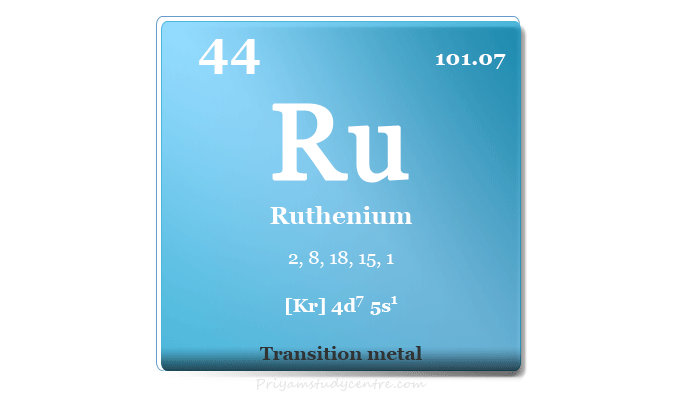
| Atomic number | 44 | ||
| Relative atomic mass | 101.07 | ||
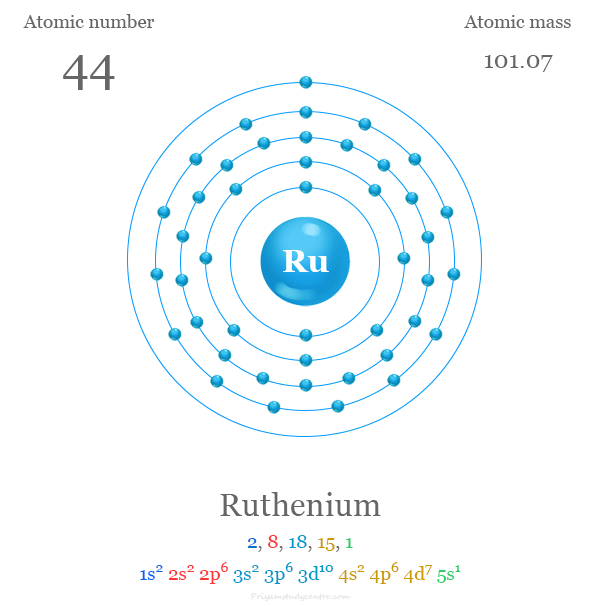
| Electron per cell | 2, 8, 18, 15, 1 | ||
| Electronic configuration | [Kr] 4d7 5s1 | ||
| Block | d-block | ||
| Group | 8 | ||
| Period | 5 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 2333° C, 2606 K | ||
| Boiling point | 4147 °C, 4420 K | ||
| Molar heat capacity | 24.06 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 12.1 g/cm3 | ||
| Electrical resistivity | 71 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.13 Å | ||
| Covalent radius | 1.36 Å | ||
| Electronegativity | 2.2 (Pauling scale) | ||
| Electron affinity | 101.31 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 710.18 | 1617.09 | 2746.94 | |
Electron Configuration
The 44 electrons in the ruthenium atom are distributed in different energy levels to show the following electron configuration given below the picture,
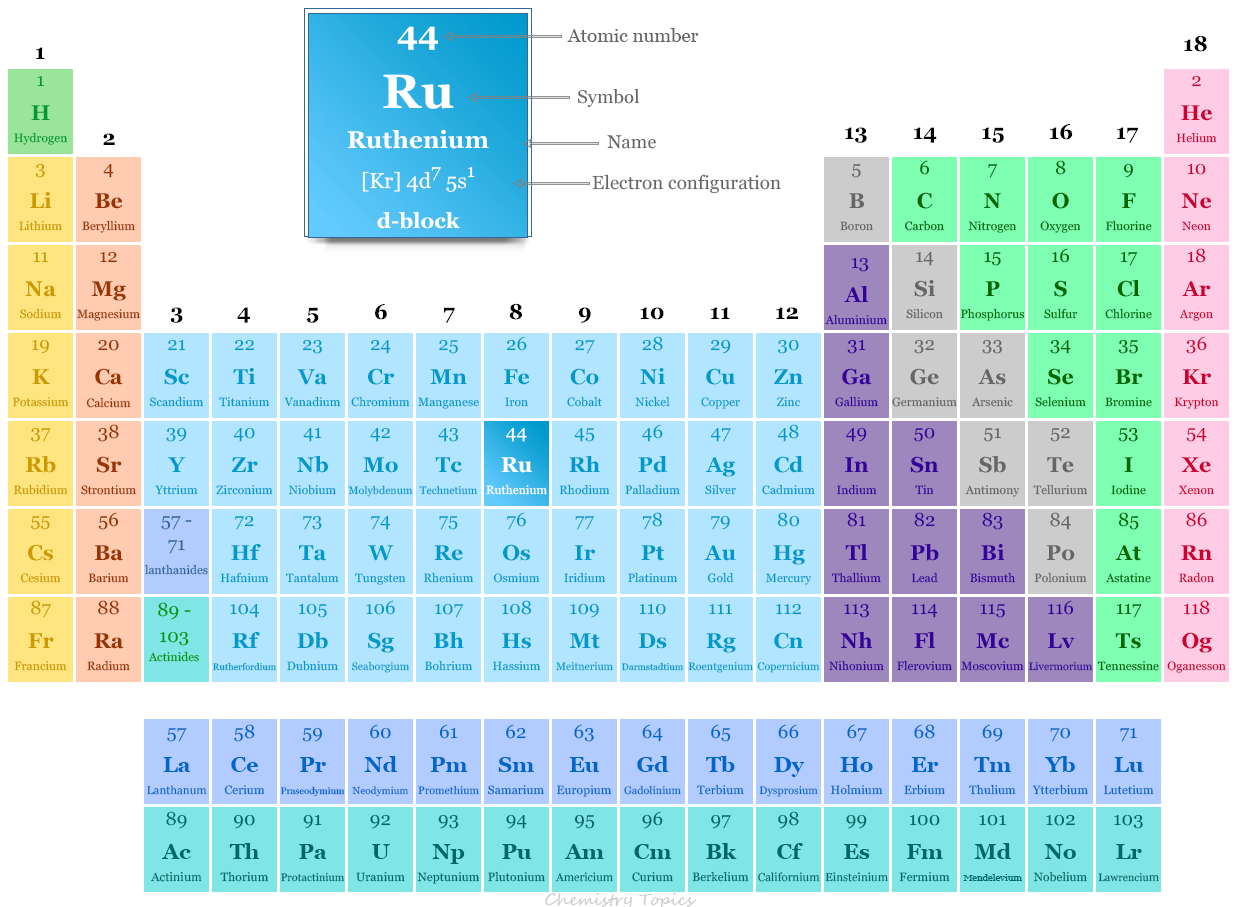
Ruthenium in the Periodic Table
Ruthenium is placed in group 8 and period 5 in the periodic table. It is a d-block element or transition metal that lies between technetium and rhodium.
Chemical Compounds
The platinum or noble metal ruthenium is quite unreactive. The metal shows the highest observed oxidation state VIII but the VI and IV states of metal are also very stable.
It forms a wide range of chemical compounds in various oxidation states. The most common oxides, fluorides, and complexes of ruthenium in +4 and +6 oxidation states are given below,
Ruthenium Oxides
The oxidation states IV and VI of Ru are quite stable. It burns in the air directly to form blue-black ruthenium dioxide with the chemical formula RuO2. The dioxide is a stable solid with a rutile structure and is insoluble in water.
The volatile orange-yellow tetroxide (RuO4) is formed by oxidation of Ru (VI) in an acid solution with KBrO3, KMnO4, Cl2, etc. The molecule contains a regular tetrahedral structure with an ozone-like odor. It is a strong oxidizing agent that dissolves in alkali by evolving oxygen gas.
Ruthenium Fluorides
The hexafluoride and tetrafluoride form of Ru is common. The brown colour hexafluoride (RuF6) is obtained by the reaction of Ru with fluorine under pressure and quenching the vapour.
It formed yellow RuF4 when RuF5 is reduced by iodine. RuF5 is a normal product of the reaction between Ru and fluorine. Probably the tetrafluoride compound is polymeric in nature.
Coordination Complexes
In lower oxidation states the metal forms a variety of coordination complexes. There are a few simple compounds observed in the +2 state of the metal. It formed numerous complexes with amine and arsine. These complexes are formed by the reduction of ruthenium metal in the presence of ligands.
Reduction of RuCl3 with hydrazine or zinc amalgam in the presence of ammonia gives dinitrogen complex [Ru(NH3)5N2]+. It is the first compound reported to contain a coordinated nitrogen molecule.
Facts About Ruthenium
- Ruthenium was the last discovered platinum group of metals that was discovered by Russian-born scientist of Baltic-German ancestry Karl Ernst Claus in 1844.
- The name of the element comes from the Latin word Ruthenia means Russia. It refers to the Ural Mountains of Russia, the original source of ores of platinum metals.
- It is the only group 8 element that does not contain 2 electrons in its outer quantum shell.
- It shows a wide range of oxidation numbers or states. The most commonly found oxidation states of metal are +8, +6, and +4.
- Like other platinum metals, it forms a wide variety of binary compounds like oxides, sulfides, and especially halides.
- It is a versatile catalyst that is used in the removal of hydrogen sulfide from oil refineries and other industrial processes. The ruthenium catalyst is also used for the production of ammonia and acetic acid.
- It is toxic to humans that believed to have carcinogenic properties. The tetroxide (RuO4) is particularly dangerous for humans.
Uses of Ruthenium
- Ruthenium is an important alloying material to impart hardness in platinum and palladium.
- It is also used as a chemical catalyst for the production of ammonia from natural gas and acetic acid from methanol.
- Ruthenium is used in the electronics industry for making resistors and electrical contacts.
- In the chlorine production process, ruthenium oxide is used to coat the anodes of electrochemical cells.
- It is added to titanium alloys to improve the corrosion resistance properties of titanium alloys.
- It is also used for alloying cobalt, molybdenum, nickel, tungsten, and other metals.
- Ruthenium compounds can be used in solar cells for the production of solar renewable energy.
- It is alloyed with platinum for the production of some jewelry.
- The beta-decaying isotope ruthenium 106 is used in radiotherapy to treat eye tumors and malignant melanoma of the uvea.
Ruthenium Price
It is used widely in the electrical industry because the properties of ruthenium are similar to that of rhodium but the price of the Ru is lower than rhodium.
The ruthenium plate is used in electrical contact and electrode base metal electroplating or sputtering. The average price of pure ruthenium metal is around $1400 per 100 grams.








