Holmium Metal
Holmium is a chemical element or rare earth metal in the periodic table with the symbol Ho and atomic number 67. Like other lanthanides, it is the reactive and softest metal that can form a yellowish oxide coating when exposed to air or oxygen. Holmium is used in solid-state laser glasses for microwave equipment and optical fiber technology. The rare earth metal holmium is also used to create a magnetic flux concentrator for making artificial magnet and laser glasses which are used in optical spectrophotometers for calibration.

It was discovered in 1878 by Swedish chemist Per Theodor Cleve and independently by Jacques-Louis Soret and Marc Delafontaine. The name of the element comes from the Latin word Holmia means the city of Stockholm.
Where is Holmium Found?
Holmium is found in nature together with other rare-earth metals. The abundance of the metal is similar to that of tungsten. Like many other lanthanides, it is found in the minerals monazite and gadolinite and extracted or separated commercially by ion-exchange chromatography techniques.
Most of the world’s production of holmium comes from the mining areas of China, the USA, Brazil, India, Sri Lanka, and Australia.
Isotopes
Naturally occurring holmium has one stable isotope with an atomic mass of 165. It also contains 35 synthetic radioactive isotopes that can be synthesized by various artificial nuclear reactions. Most of these radioactive isotopes have half-lives of less than 3 hours.
The metastable 166m1Ho (half-life of around 1200 years) has been used in nuclear physics for the calibration of gamma-ray spectrometers.
Properties
Holmium is a soft, malleable, lustrous metal with high electrical conductance. It is a silvery colour metal belonging to the lanthanide series of the periodic table.
| Holmium | |||
| Symbol | Ho | ||
| Discovery | Per Teodor Cleve and independently by Marc Delafontaine and Louis Soret in 1878 | ||
| Name derived from | The Latin name for Stockholm means Holmia | ||
| Common isotope | 67Ho165 | ||
| Oxidation state | +3 | ||
| CAS number | 7440-60-0 | ||
| Periodic properties | |||
| Atomic number | 67 | ||
| Relative atomic mass | 164.930 | ||
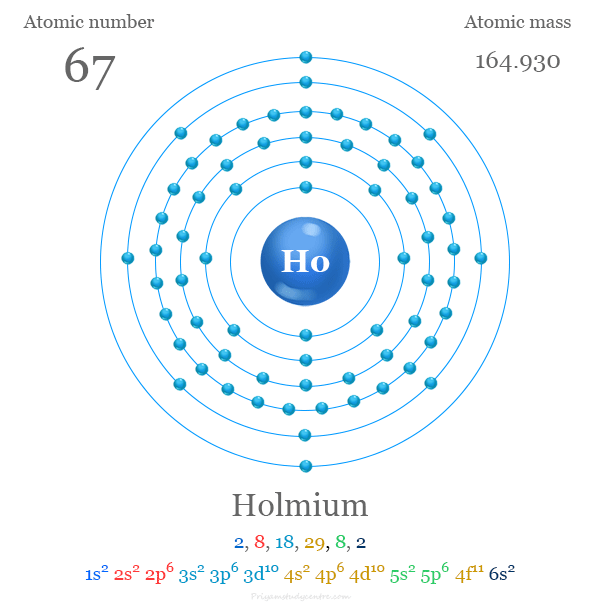
| Electron per cell | 2, 8, 18, 29, 8, 2 | ||
| Electronic configuration | [Xe] 4f11 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1472 °C, 1745 K | ||
| Boiling point | 2700 °C, 2973 K | ||
| Molar heat capacity | 27.15 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 8.80 g/cm3 | ||
| Heat of fusion | 17 kJ mol−1 | ||
| Heat of vaporization | 251 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.30 Å | ||
| Covalent radius | 1.79 Å | ||
| Electronegativity | 1.23 (Pauling scale) | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 580.99 | 1138.50 | 2203.73 | |
Holmium Electron Configuration
The 67 electrons of holmium are distributed in different energy levels or orbitals to give the following electronic configuration,
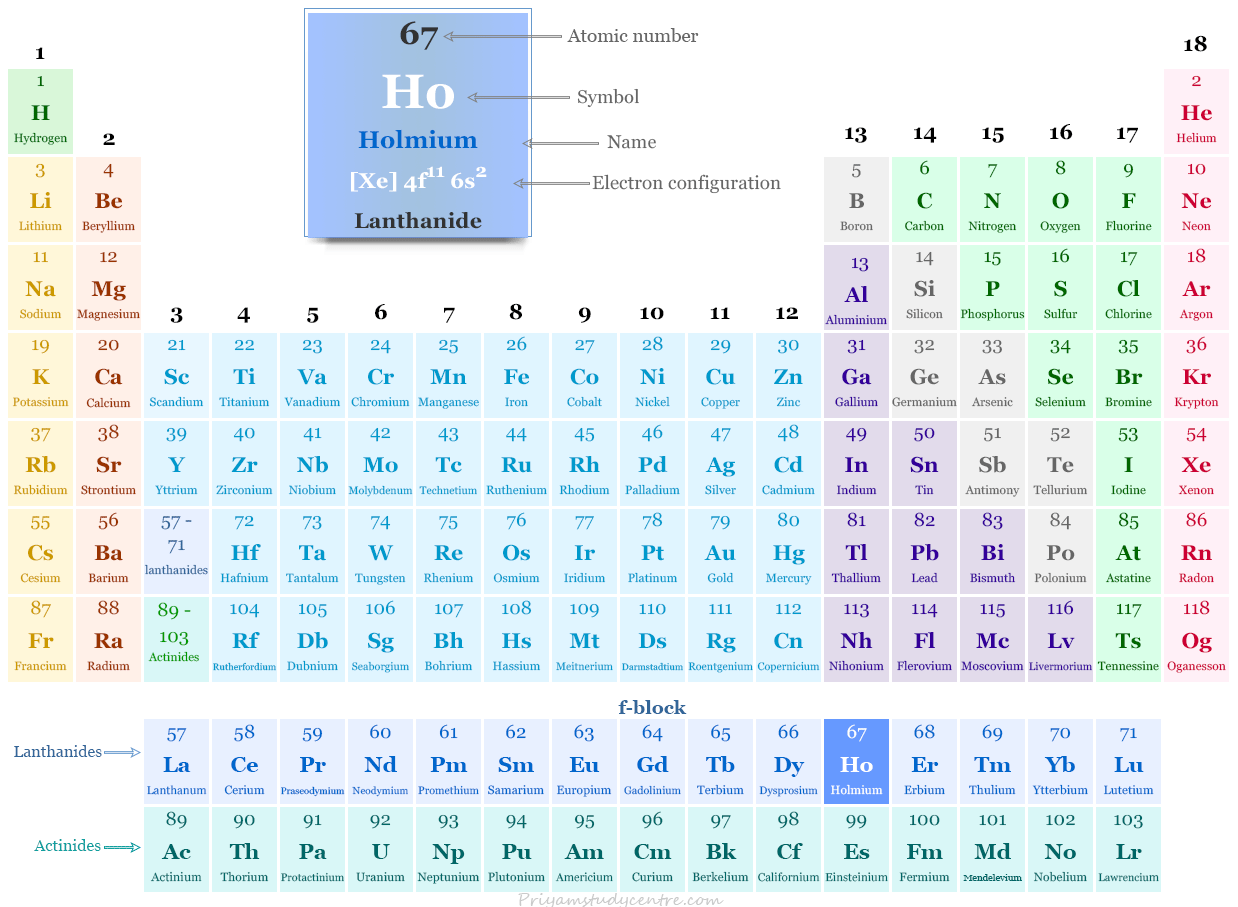
Holmium in the Periodic Table
The rare earth metal holmium is placed in the f-block of the periodic table. It is lanthanide that lies between dysprosium and erbium.
Chemical Properties
Holmium is a highly reactive and electropositive rare earth metal that is stable in dry air at ordinary temperatures but rapidly dull in humid atmospheres. It burns in the air to form yellow oxide Ho2O3.
4 Ho + 3 O2 → 2 Ho2O3
It stands far above hydrogen in the electrochemical series. Therefore, it reacts slowly with cold water and rapidly with hot water to liberate hydrogen gas.
2 Ho + 6 H2O → 2 Ho(OH)3 + 3 H2
Reactions with acids are more vigorous but the metal does not dissolve in alkalies. It dissolves readily in dilute sulfuric acid to form solutions containing the yellow Ho (III) ions.
2 Ho + 3 H2SO4 → 2 Ho3+ + 3 SO4−2 + 3 H2
Holmium is normally from trihalides with halogens like fluorine, chlorine, bromine, and iodine.
2 Ho + 3 X2 (X = F, Cl, Br, I) → 2 HoX3
Facts About Holmium
- It is not found in nature in free elements due to its high reactivity.
- It is one of the rare lanthanides that is 20 times more abundant than silver.
- It is the sixth most volatile lanthanide after ytterbium, europium, samarium, thulium and dysprosium.
- Like many lanthanides, it forms chemical compounds in the +3 oxidation number or state but in the +2 state, it forms fluoride (HoF3) and chloride (HoCl3) compounds.
- Holmium is used to make a strong magnet, solid-state laser glasses, and optical fiber. When holmium is combined with yttrium to form a compound that is used for making a strong magnet.
- It does not play any biological role in human beings but holmium salts can simulate the metabolism of human beings.
What is Holmium Used for?
- Holmium is alloyed with other metals to make the strongest artificial magnet.
- It is used to control chain reactions in nuclear power reactors because it has high neutron absorption properties.
- Holmium-doped yttrium iron garnet (YIG) and yttrium lithium fluoride (YLF) have been used for making solid-state laser glasses. Such laser glasses are used widely in microwave and optical fiber technology.
- The laser glasses containing holmium oxide have sharp optical absorption peaks with a wavelength range of 200–900 nm. Therefore, it can be employed in optical spectrophotometers for the purpose of calibration.
- The long-lived metastable radioactive isotope of holmium (166m1Ho) is used for the calibration of gamma-ray spectrometers.