Yttrium Metal
Yttrium is a silvery white transition metal of group 3 of the periodic table with the symbol Y and atomic number 39. It is chemically similar to rare earth metals but placed with the d-block element in the periodic table. Yttrium is used as an additive in aluminium and magnesium alloys and an enhancer for metals chromium, molybdenum, titanium, and zirconium. It is also used in the production of various types of synthetic garnets such as yttrium iron garnets and yttrium, iron, aluminium, and gadolinium garnets.

Yttrium oxide was identified by Finnish chemist Johan Gadolin in 1789 from Carl Axel Arrhenius heavy black rock sample. The complete analysis of the sample was published in 1794. The name of the metal was derived from the Swedish village of Ytterby.
Isotopes
Naturally, it has only one isotope 89Tb. It also contains 35 unstable radioactive isotopes that can be prepared by various artificial nuclear reactions. The isotope yttrium-90 is used in medicine for the treatment of cancers.
These isotopes are the most common nuclear fission products of uranium during nuclear explosions or nuclear reactor operations. The most important isotopes 91Y and 90Y are obtained during radioactive pollution or waste management.
88Y (half-life of 106.6 days) and 91Y (half-life of 58.51 days) are the most stable radioisotopes of yttrium. Most of the other isotopes have half-lives of less than a day. The primary radioactive decay mode of isotopes is electron capture or beta decay.
Properties
The properties of the metal are similar to that of lanthanides or rare earth metals. It is found always with rare earth minerals.
One of the most important differences between the chemistry of yttrium and other lanthanides or rare earth metals is that it mostly shows a +3 oxidation number or state but half the lanthanides can have shown oxidation number other than three.
| Yttrium | |||
| Symbol | Y | ||
| Discovery | Johan Gadolin in 1794 | ||
| Name derived from | It is named after Ytterby, Sweden | ||
| Common isotope | 39Y89 | ||
| Oxidation states | +3 | ||
| CAS number | 7440-65-5 | ||
| Periodic properties | |||
| Atomic number | 39 | ||
| Relative atomic mass | 88.906 | ||
| Electron per cell | 2, 8, 18, 9, 2 | ||
| Electronic Configuration | [Kr] 4d1 5s2 | ||
| Block | d-block | ||
| Group | 3 | ||
| Period | 5 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1522 °C, 1795 K | ||
| Boiling point | 3345 °C, 3618 K | ||
| Molar heat capacity | 26.53 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 4.47 g/cm3 | ||
| Magnetic Ordering | paramagnetic | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.32 Å | ||
| Covalent radius | 1.76 Å | ||
| Electronegativity | 1.22 (Pauling scale) | ||
| Electron affinity | 29.621 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 599.88 | 1179.44 | 1979.88 | |
Electron Configuration
The 39 electrons of yttrium are distributed in different energy levels to show the following electronic configuration given below the picture,
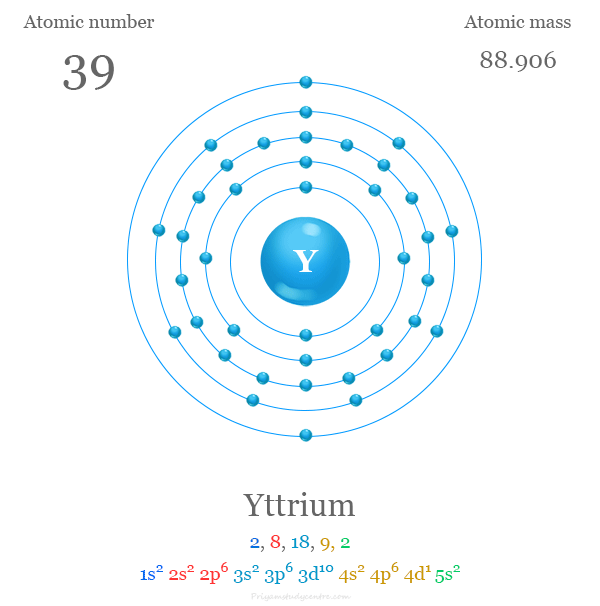
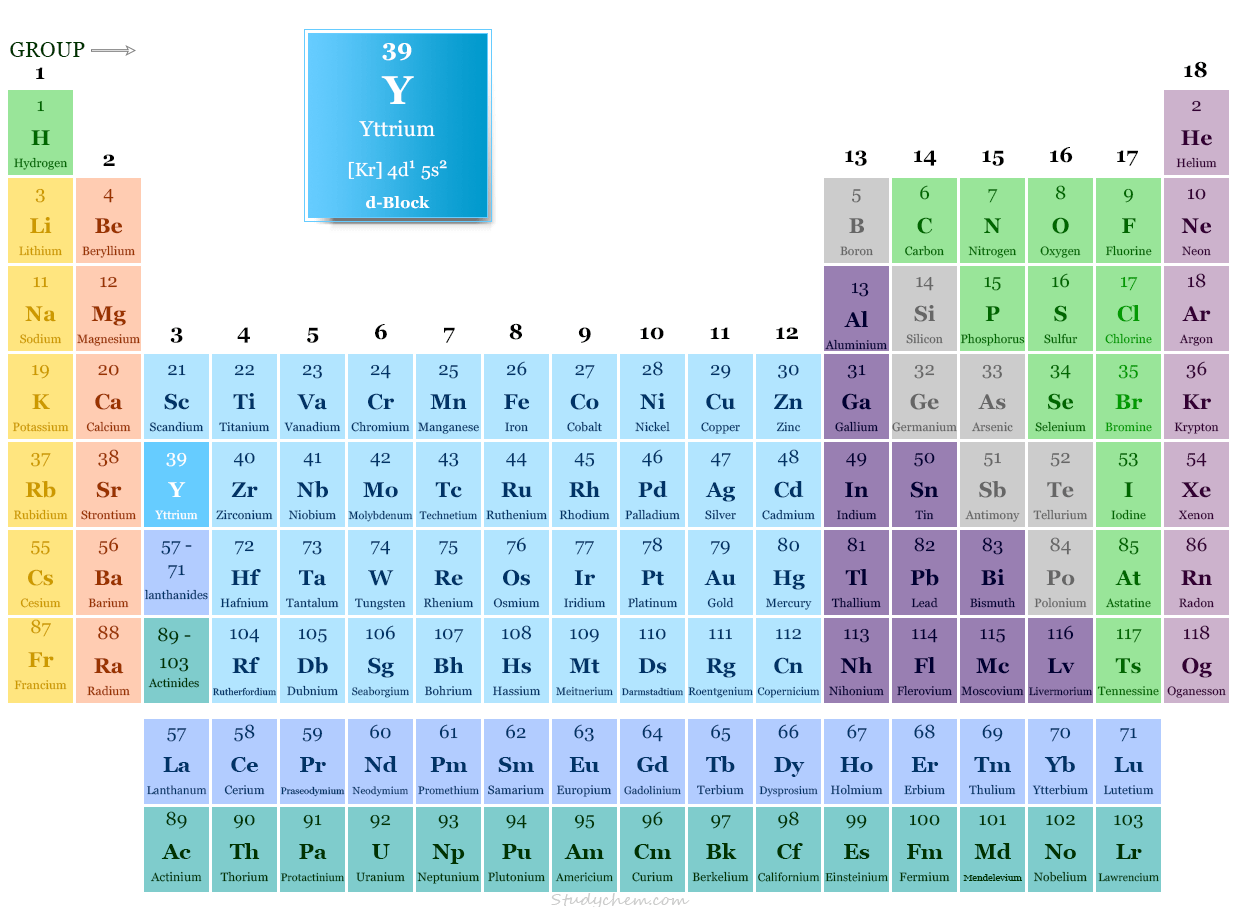
Yttrium in the Periodic Table
It is placed in group 3 and period 5 of the periodic table. It is a transition metal or rare earth metal that lies between strontium and zirconium.
Chemical Properties
The highly crystalline yttrium metal is fairly stable in the air but readily oxidized when heated. It readily reacts with water to form Y (III) oxide (Y2O3) or yttria. Concentrated nitric acid or hydrofluoric acid does not rapidly attack Y metal. Strong sulfuric acid rapidly attacks to form yttrium sulfate.
Commonly, all three valence electrons of the Y atom participate in chemical bonding to form various types of chemical compounds. The fluoride, hydroxide, and oxalate of yttrium are water-insoluble compounds but bromide, chloride, iodide, nitrate, and sulfate are water-soluble compounds.
It reacts with halogens like fluorine, chlorine, and bromine to form corresponding trihalides. At elevated temperatures, carbon, phosphorus, selenium, silicon, and sulfur react with yttrium to form corresponding binary compounds of the metal.
Facts About Yttrium
- It is a soft, silver-white, lustrous, and highly crystalline solid transition metal in group 3 of the periodic table.
- It is the first element of the 5d series.
- The bulk from the pure metal is relatively stable in the air due to the formation of a protective layer on its surface.
- The finely divided powder of yttrium is very unstable in the air.
- The Y3+ ion is colorless in solution due to the absence of d and f electrons.
- It formed nitride (YN) when heated to 1000 °C in the presence of nitrogen.
- The metal is always found in nature together with rare-earth minerals.
- It may be separated from other rare earth metals by ion exchange chromatography or solvent extraction process.
- It can not perform any biological role on living organisms.
- The metal is highly toxic for humans, other animals, and plants. The intake of yttrium compounds may cause lung disease in humans.
Uses of Yttrium
- Yttria (Y2O3) or yttrium oxide sulfide (Y2O2S) doped with europium cation (Eu3+) are used as a red phosphors component for making color television cathode ray tubes.
- The rare earth metal is used as an additive to increase the strength of aluminium and magnesium alloys.
- It is used as an enhancer for metals chromium, molybdenum, titanium, and zirconium.
- Yttrium compounds are used as a chemical catalyst in the ethylene polymerization process.
- Y-Fe garnets are very useful in microwave filters while yttrium-aluminium garnet is used for making lasers and white LED lights.
- In medicinal chemistry, the radioactive isotope yttrium-90 can be used for the treatment of cancers such as lymphoma, leukemia, liver, ovarian, colorectal, pancreatic, and bone cancer.
- A small quantity of yttrium is used in the electrode of lithium iron phosphate battery (LFP).
- Yttrium oxide is used in some ceramics applications and glass-making processes.








