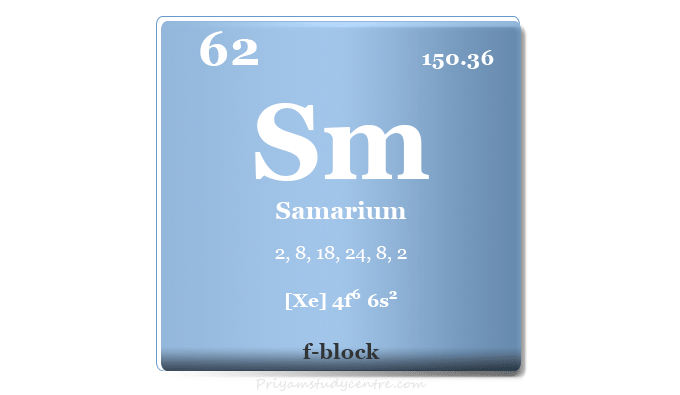
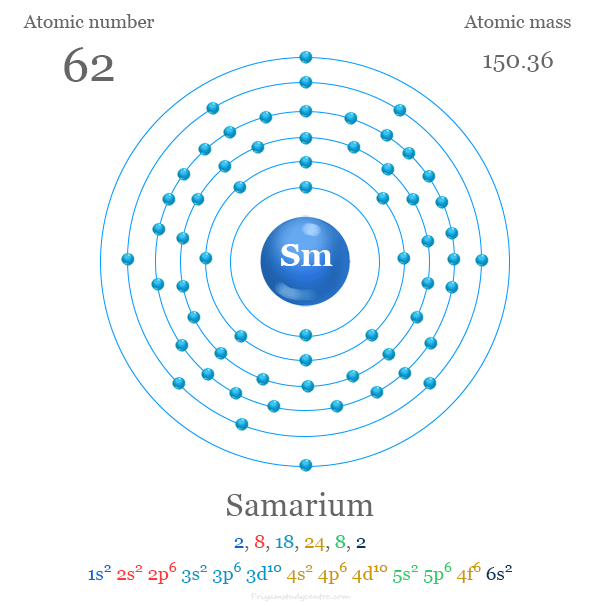
Samarium Element
Samarium is a chemical element or rare earth metal in the periodic table with the symbol Sm and atomic number 62. It is a soft, ductile, and silvery-white rare earth metal that lies with f-block elements or lanthanides. The density and hardness of the samarium element are similar to that of zinc. Like neodymium, it is alloyed with cobalt to form samarium-cobalt magnets. It is also used in X-ray lasers, and glasses, as a catalyst for alcohol production, nuclear medicine, and as an absorber in nuclear power reactors. The radioactive isotope samarium-153 is an active component of radioactive medicine. In medicinal chemistry, Sm-153 is used for the treatment of lung cancer, prostate cancer, breast cancer, and osteosarcoma. Another isotope, Sm-149 is a strong neutron absorber. Therefore, it is added to the control rods of nuclear power reactors.
Where is Samarium Found?
It is the 40th most abundant element in earth’s crust and the 5th most abundant element among the lanthanides family. Samarium is found along with other rare earth metals in various minerals. The principal minerals of Sm are monazite and bastnaesite.
The most abundant commercial sources of samarium are China, India, Brazil, the US, Australia, and Sri Lanka.
It can be separated from the other rare earth metals by ion exchange chromatography and solvent extraction method. Recently, the electrochemical deposition technique has been used to separate samarium from other lanthanides.
Isotopes
Naturally occurring rare earth metal samarium contains five stable isotopes with mass numbers 144, 149, 150, 152, and 154. Artificial nuclear reactions may be synthesized various radioactive isotopes of Sm. Some isotopes are obtained by the radioactive decay of neodymium isotopes.
It contains two extremely long-lived radioactive isotopes 147Sm (half-life t1/2 = 1.06×1011 years) and 148Sm (7×1015 years). These two isotopes are obtained primarily by alpha decay to neodymium. Lighter unstable isotopes are obtained by electron capture to promethium.
The radioactive isotope, Sm-153 is used to kill cancer cells in the lungs, prostate, breast, and osteosarcoma. Another isotope, Sm-149 is used as a strong neutron absorber in control rods of nuclear reactors.
Properties
Samarium is a silvery white rare earth metal belonging to the lanthanide family of the periodic table. It is relatively stable at room temperature but ignites when heated at 150 °C. It forms an oxide layer in moist air.
Like rare earth metal europium, samarium has also shown a stable +2 oxidation state along with a +3 state.
| Samarium | |||
| Symbol | Sm | ||
| Discovery | Paul-Émile Lecoq de Boisbaudran in 1879 | ||
| Name derived from | The mineral samarskite which was used for the first isolation | ||
| Common isotope | 62Sm152 | ||
| Oxidation states | +3, +2 | ||
| CAS number | 7440-19-9 | ||
| Periodic properties | |||
| Atomic number | 62 | ||
| Relative atomic mass | 150.36 | ||
| Electron per cell | 2, 8, 18, 24, 8, 2 | ||
| Electronic Configuration | [Xe] 4f6 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1072 °C, 1345 K | ||
| Boiling point | 1794°C, 2067 K | ||
| Molar heat capacity | 29.54 J mol−1 K−1 | ||
| Crystal structure | rhombohedral | ||
| Density | 7.52 g/cm3 | ||
| Heat of fusion | 8.62 kJ mol−1 | ||
| Heat of vaporization | 192 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.36 Å | ||
| Covalent radius | 1.85 Å | ||
| Electronegativity | 1.17 (Pauling scale) | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 544.53 | 1068.09 | 2257.80 | |
Electron Configuration
The 62 electrons of the samarium are arranged to show the electron configuration of [Xe] 4f6 6s2. Like europium, Sm commonly uses two or three valence electrons to show +2 or +3 oxidation numbers or states.
Samarium in the Periodic Table
It is placed with lanthanide in the periodic table. Samarium is a costly rare earth metal that lies between promethium and europium.
Chemical Properties
Samarium is a highly electropositive and active metal. The electrode potential value of Sm is comparable to that of alkaline earth metals. It acts as a strong reducing agent.
The compact rare earth metal is quite stable to dry air at ordinary temperatures but rapidly dull in a humid atmosphere. Samarium metal tarnishes slowly in the air by forming an oxide layer.
Like other rare earth metals, it also burns in oxygen at 150 °C to form Sm (III) oxide.
4 Sm + 3 O2 → 2 Sm2O3
Samarium is attacked by acids like dilute sulfuric acid with the liberation of hydrogen gas. The acidic solutions contain pale green Sm3+ ions.
2 Sm + 3 H2SO4 + 16 H2O → 2 [Sm(H2O)9]+3 + 3 SO4−2 + 3 H2
Facts About Samarium
- The most common oxidation state of samarium is +3 but like europium +2 state is also common.
- Samarium is the hardest and the most brittle of the rare earth elements that tarnish in air and ignite in oxygen at 150 °C to form Sm oxide.
- Under ordinary conditions, the metal has rhombohedral crystals but after heating the crystal structure to hexagonal close-packed (hcp). Further heating, Sm formed a body-centered cubic (bcc) phase.
- It is used to make samarium-cobalt permanent magnets, samarium X-ray lasers, glasses, a catalyst for alcohol production, pain treatment medicine for bone cancer, and an absorber in nuclear reactors.
- It is a costly rare earth metal. An approximate price of pure samarium is about $360 per 100 grams of Sm metal.
- It may be separated from minerals monazite and bastnasite using ion exchange chromatography and solvent extraction methods. Electrolysis may also be used for the production of pure samarium metal from its molten chloride with sodium chloride.
- The low toxic samarium metal has no known biological role.
Chemical Compounds
It is one of the few lanthanides that shows an oxidation state of +2 along with a +3 state. The aqueous solution Sm2+ ions show blood-red colour.
Samarium Oxides
The most common and stable oxide of Sm is Sm2O3. Samarium metal also forms monoxide with the chemical formula SmO. The metal tarnishes readily in the humid air by forming an Sm (III) oxide layer.
The oxide is obtained by burning the metal in air or oxygen at a temperature above 150 °C.
4 Sm + 3 O2 → 2 Sm2O3
It is also obtained by thermal decomposition of samarium (III) carbonate, hydroxide, nitrate, oxalate, or sulfate.
Sm2O3 is commonly white to off-yellow in colour and used in optical and infrared absorbing glass to absorb infrared radiation. It is also used as a neutron absorber in control rods of nuclear power reactors.
Samarium Hydroxide
It is an important chemical compound of samarium with the chemical formula Sm(OH)3. It forms samarium (III) salts when reacts with sulfuric acid.
2 Sm(OH)3 + 6 H2SO4 → Sm2(SO4)3 + 6 H2O
It is potentially used in electronics, optics, and catalysis.
Halides
The metal Sm reacts with all the halogens like fluorine, chlorine, bromine, and iodine to form trihalides.
2 Sm + 3 X2→ 2 SmX3 (X = F, Cl, Br or I)
The dihalides are obtained by further reduction of trihalides with samarium, lithium, or sodium at elevated temperatures (about 700–900 °C). In addition to dihalides, the reduction also produces various non-stoichiometric halides like Sm3F7, Sm14F33, Sm27F64, Sm11Br24, Sm5Br11, and Sm6Br13.
Uses of Samarium
It is used widely for making samarium-cobalt magnets. It may also be used in various catalysis processes, absorbers in nuclear reactors, medicine for cancer treatment, making glasses, etc.
Samarium-Cobalt Magnets
It is used for making samarium-cobalt magnets with the chemical formula SmCo5 or Sm2Co17. Such types of magnets are much more powerful than iron magnets.
Samarium-cobalt magnets are used commonly in small motors, headphones, and high-end magnetic pickups for guitars and related musical instruments. Presently, due to the high price of Sm, these magnets are replaced by neodymium magnets.
It is suitable only for high-temperature applications. They show magnetic properties at high temperatures. Therefore, these magnets are used in microwaves.
In Catalysis
Samarium and its compounds are very useful in catalysis processes.
- These catalysts help to decompose pollution products such as plastics and polychlorinated biphenyls (PCB).
- They are also useful for the dehydration and dehydrogenation of ethanol.
- Sm (III) triflate is an effective Lewis acid catalyst that is used in halogen-promoted Friedel–Crafts reaction with alkenes.
- Sm (II) iodide is a very common reducing and coupling agent that uses for the synthesis of various organic compounds.
In Medicine
The beta emitter samarium-153 (half-life 46.3 hours) is used to kill cancer cells in lung cancer, prostate, breast, and osteosarcoma.
In quadramet medication, Sm-153 is chelated with ethylene diamine tetramethylene phosphonate (EDTMP) and injected intravenously. The chelation prevents the accumulation of radioactive samarium in our body but beta radiation can kill cancer cells of lung cancer, prostate cancer, breast cancer, and osteosarcoma.
In Nuclear Reactors
Samarium-149 has a high cross-section for neutron capture. Therefore, it is used in control rods of nuclear reactors due to the stability of absorption compared to other materials such as boron and cadmium. Most of the nuclear fusion and decay products of 149Sm are other isotopes of samarium and are also good neutron absorbers.
Other Applications
- It is used for making carbon arc lamp electrodes for studio lighting and projection.
- The off-yellow colour Sm2O3 is used in optical and infrared absorbing glass to absorb infrared radiation.
- A minor quantity of Sm is a part of mischmetal that is used in the ignition devices of many lighters and torches.
- Samarium-doped crystalline solids like calcium chloride or calcium are used for making optical lasers.