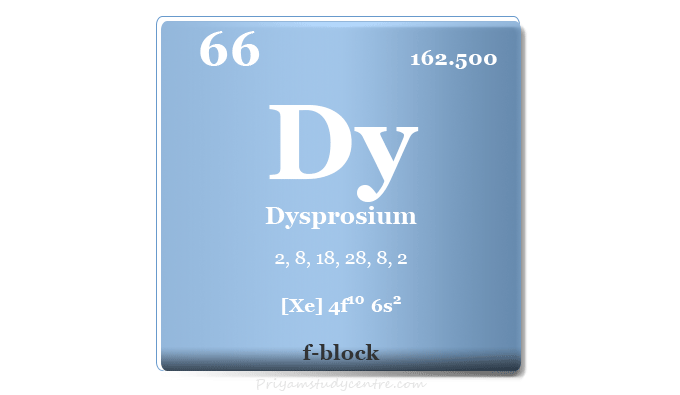
Dysprosium Element
Dysprosium is a chemical element or rare earth metal in the periodic table with the symbol Dy and atomic number 66. Pure dysprosium is a relatively hard and silvery white rare earth metal. It is quite stable in the air and oxidized in the presence of oxygen. The application of a pure form of dysprosium is very little. The metal and its compounds are used mainly for improving the workability of neodymium–iron–boron magnets, neutron-absorbing materials for control rods in nuclear power reactors, laser materials, and commercial lighting.
Dysprosium was first identified in 1886 by French chemist Paul Émile Lecoq de Boisbaudran. The pure form of the element was not isolated before the discovery of ion exchange chromatography techniques. The name of the rare earth metal was given from the Greek word dysprositos means hard to get.
Where is Dysprosium Found?
It is never found in nature as a free element and no dysprosium-dominant mineral has been found yet in the earth’s crust. Like other various lanthanides, dysprosium is found in the minerals monazite and bastnaesite. It may also be found in smaller quantities in xenotime and fergusonite.
It can be extracted or separated from rare earth minerals by ion exchange chromatography and solvent extraction process. Today, it is primarily synthesized through an ion exchange process from monazite sand.
Isotopes
Naturally occurring dysprosium has seven isotopes with atomic masses ranging from 156 to 164. It also contains 29 radioactive isotopes that can be synthesized by various artificial nuclear reactions. The atomic masses of these radioactive isotopes range from 138 to 173. It also contains 11 metastable isomers.
The most stable radioactive isotope is 154Dy (half-life 3×106 years) while the least stable radioactive isotope is 138Dy (half-life 200 ms). The primary radioactive decay mode of dysprosium isotopes is electron capture, alpha decay or beta decay.
In medicinal chemistry, the isotope 165Dy is used in arthritis therapy or radiosynovectomy.
Properties
Dysprosium is a silvery white soft solid at room temperature. It is oxidized in air to form an oxide layer. The metal is soft enough to cut by a knife and readily attacked and dissolved by dilute and concentrated mineral acids like sulfuric acid, nitric acid, or hydrochloric acid.
| Dysprosium | |||
| Symbol | Dy | ||
| Discovery | Paul-Émile Lecoq de Boisbaudran in 1886 | ||
| Name derived from | The Greek word dysprositos means hard to get | ||
| Common isotope | 66Dy164 | ||
| Oxidation states | +3 | ||
| CAS number | 7429-91-6 | ||
| Periodic properties | |||
| Atomic number | 66 | ||
| Relative atomic mass | 162.500 | ||
| Electron per cell | 2, 8, 18, 28, 8, 2 | ||
| Electronic Configuration | [Xe] 4f10 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1412 °C, 1685 K | ||
| Boiling point | 2567 °C, 2840 K | ||
| Molar heat capacity | 27.7 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 8.55 g/cm3 | ||
| Heat of fusion | 11.06 kJ mol−1 | ||
| Heat of vaporization | 280 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.31 Å | ||
| Covalent radius | 1.80 Å | ||
| Electronegativity | 1.22 (Pauling scale) | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 573.02 | 1125.98 | 2199.90 | |
Electron Configuration of Dysprosium
The 66 electrons of dysprosium are distributed in different orbitals to give the following electronic configuration,
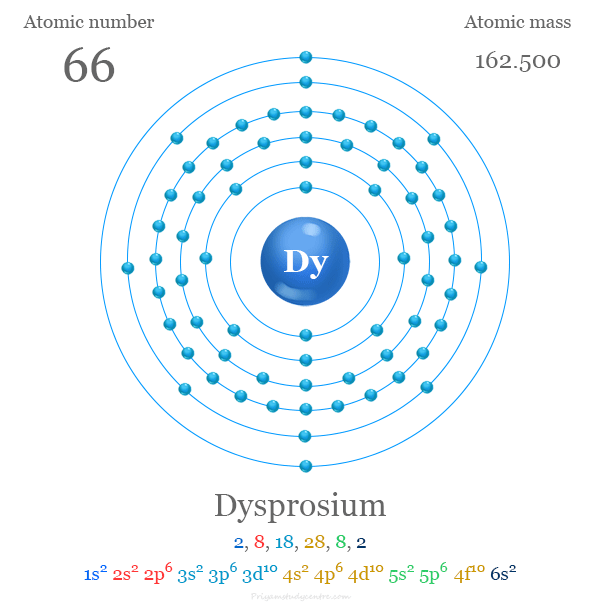
The valence shell electron configuration of the metal is [Xe] 4f10 6s2. Like other lanthanides, it commonly uses three electrons for chemical bonding purposes to show a +3 oxidation number or state.
Dysprosium in the Periodic Table
The rare earth metal dysprosium is placed with the f-block elements of the periodic table. It is lanthanide that lies between terbium and holmium.
Chemical Properties
It is relatively stable in air at room temperature but in a humid atmosphere, it forms a Dy (III) oxide layer.
4 Dy + 3 O2 → 2 Dy2O3
Pure metal is quite an electropositive and hazardous element for the environment because it reacts with water to form flammable hydrogen gas.
2 Dy + 6 H2O → 2 Dy(OH)3 + 3 H2
It decomposes to form DyO(OH) at elevated temperatures but decomposes again to form Dy (III) oxide.
It dissolves readily in dilute sulfuric acid to form solutions that contain yellow Dy (III) ions. The chemical reaction formed paramagnetic dysprosium (III) sulfate.
2 Dy + 3 H2SO4 → 2 Dy3+ + 3 SO4−2 + 3 H2
Facts About Dysprosium
- Paul Lecoq de Boisbaudran identified dysprosium in 1886 but pure metal cannot be isolated before the 1950s.
- The abundance of rare earth metal dysprosium is 5.2 mg/kg in the Earth’s crust and 0.9 ng/L in seawater.
- Small amounts of impurities can greatly affect the physical properties of rare earth metal Dy.
- It is used widely in the production of neodymium-based magnets due to its ferromagnetic properties at temperatures below 85K.
- Dysprosium powdered and thin foil can explode in the presence of a spark and the fire cannot be extinguished by using water.
- The soluble salts of the metal are mildly toxic while the insoluble salts are non-toxic in nature.
- The nitrate of dysprosium will ignite upon contact with human skin and other organic compounds.
- It can not be performed in any biological role in human beings or other animals.
- The price of the pure form of dysprosium is about $450 to $500 per 100g.
Uses of Dysprosium
- Dysprosium uses mainly in alloys of neodymium-based magnets. Usually, 6% of neodymium is replaced by dysprosium in neodymium–iron–boron magnets. These magnets are commonly used for making wind turbines and electric vehicles. It is resistant to demagnetization properties at high temperatures.
- It is used in the data storage system because Dy has high magnetic susceptibility.
- Dysprosium is also useful to improve the corrosion resistance properties of magnets.
- Terfenol-D contain iron and terbium with dysprosium.
- It is used in control rods of nuclear power reactors due to the absorbing capability of neutrons.
- The conjunction of rare earth metal with vanadium and other elements is used for making laser materials and commercial lighting.
- It doped yttrium aluminium garnet excited in the ultraviolet region of the electromagnetic spectrum. It results in the emission of photons of longer wavelengths in the visible region.
- The iodide and bromide of dysprosium are used for making high-intensity metal-halide lamps.