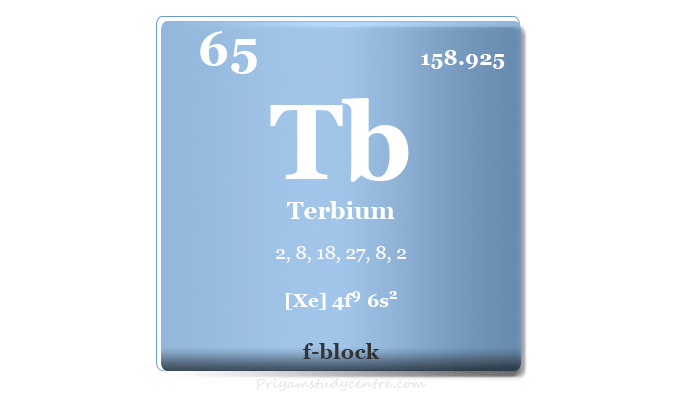
Terbium Element
Terbium is a chemical element or rare earth metal in the periodic table with the symbol Tb and atomic number 65. It is the reactive and softest lanthanide that is readily oxidized by atmospheric oxygen or moisture. Terbium is used in solid-state devices by doping it with calcium fluoride, calcium tungstate, and strontium molybdate. It is also used for making color tv tubes, low-energy lightbulbs, mercury lamps, semiconductor devices, and trichromatic lighting technology. Terbium is alloyed with dysprosium and iron to maintain the strength of the magnetic field.
It was discovered in 1843 by Swedish chemist Carl Gustaf Mosander from an impurity in yttrium oxide (Y2O3). The name of the f-block element terbium was given after the village of Ytterby in Sweden. The pure form of Tb was not isolated until the discovery of ion exchange chromatography techniques.
Where is Terbium Found?
Terbium is never found free in nature. It can be found in many minerals of other rare earth elements but no Tb-dominant mineral has yet been known.
It may be separated from minerals monazite and bastnaesite by ion exchange chromatography and solvent extraction. The main mining areas of terbium-rich minerals are China, the USA, India, Sri Lanka, Brazil, and Australia.
Isotopes
Naturally occurring terbium has only one isotope 159Tb. It also contains 36 radioactive isotopes that can be prepared by various artificial nuclear reactions. The majority of these radioactive isotopes have half-lives shorter than a minute.
The heaviest radioactive isotope of terbium is 171Tb and the lightest is 135Tb. The primary radioactive decay mode of terbium isotopes is electron capture or beta decay.
Properties
It is a soft, malleable, ductile, silver-gray metal that is stable in dry air but oxidized slowly in humid air. It is soft enough to cut by a knife.
| Terbium | |||
| Symbol | Tb | ||
| Discovery | Carl Gustav Mosander in 1843 | ||
| Name derived from | Named after the mineral Ytterby found in Sweden | ||
| Common isotope | 65Tb159 | ||
| Oxidation states | +4, +3 | ||
| CAS number | 7440-27-9 | ||
| Periodic properties | |||
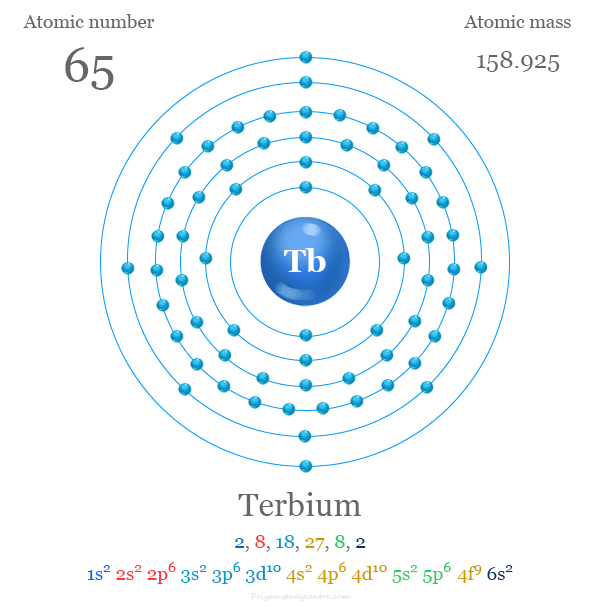
| Atomic number | 65 | ||
| Relative atomic mass | 158.925 | ||
| Electron per cell | 2, 8, 18, 27, 8, 2 | ||
| Electronic Configuration | [Xe] 4f9 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1359 °C, 1632 K | ||
| Boiling point | 3230 °C, 3503 K | ||
| Molar heat capacity | 28.91 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 8.23 g/cm3 | ||
| Heat of fusion | 10.15 kJ mol−1 | ||
| Heat of vaporization | 391 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.33 Å | ||
| Covalent radius | 1.81 Å | ||
| Electronegativity | Unknown | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 565.77 | 1111.51 | 2113.99 | |
Terbium in the Periodic Table
The rare earth metal terbium is placed in the f-block of the periodic table. It is a lanthanide that lies between gadolinium and dysprosium.
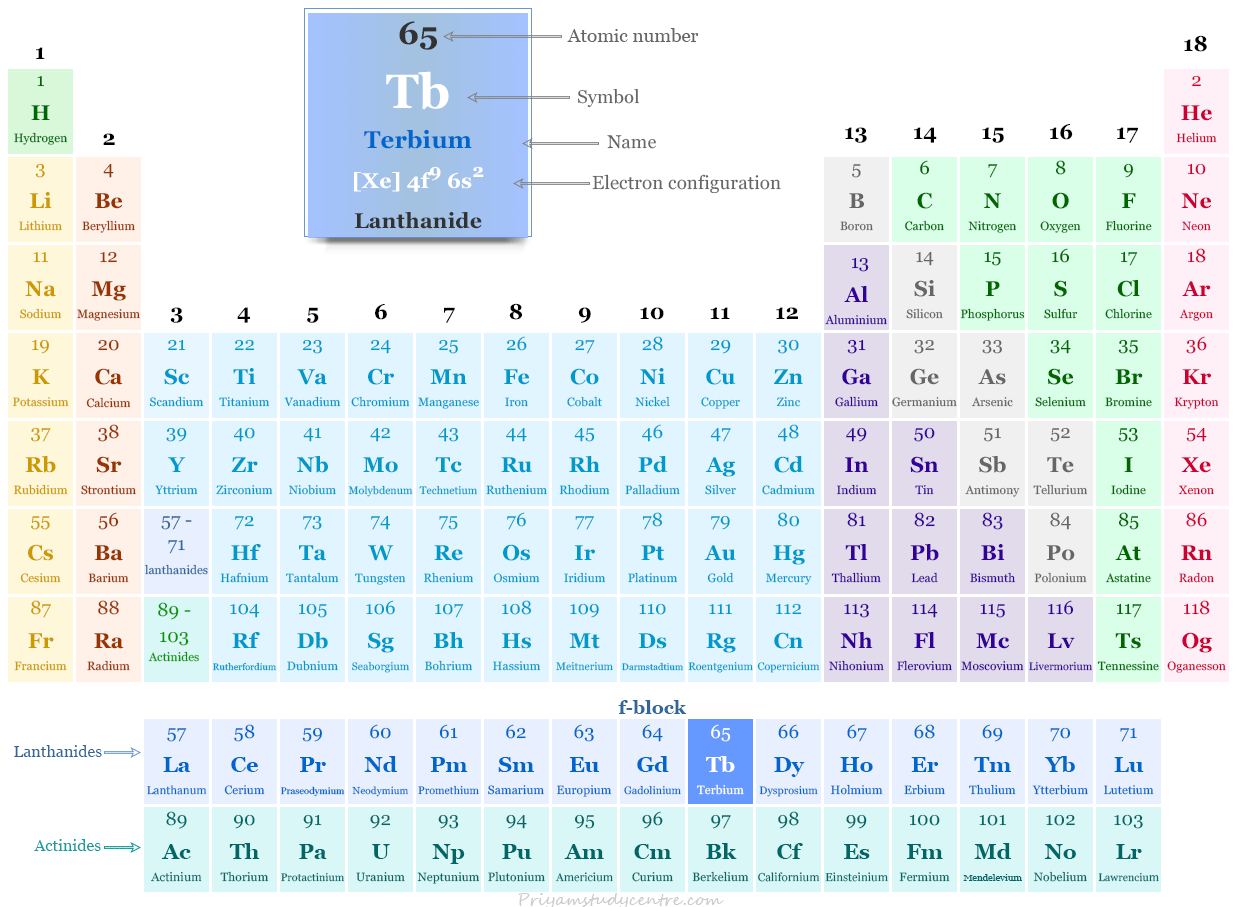
Electronic Configuration of Terbium
The 65 electrons of a terbium atom are arranged to show the electronic configuration of [Xe] 4f9 6s2. Generally, it shows a +3 oxidation number or state by losing three electrons.
The stability of the half-filled [Xe] 4f7 configuration allows further ionization of a fourth electron to show a +4 oxidation number or state.
Chemical Properties
Like other lanthanides, terbium behaves as an active metal. It combines most of the periodic table elements like metals and non-metals to form Tb (III) and Tb (IV) derivatives. It combines with nitrogen, carbon, sulfur, phosphorus, boron, selenium, silicon, and arsenic at elevated temperatures to form their respective binary compounds.
Metallic terbium is relatively stable in dry air but it tarnishes quickly in a humid atmosphere to form an oxide layer. It also oxidizes in air to form a mixed Tb (III, IV) oxide.
4 Gd + 3 O2 → 2 Gd2O3
8 Tb + 7 O2 → 2 Tb4O7
The electropositive metal terbium dissolves slowly in cold water but quite quickly in warm water by the liberation of hydrogen gas and formation of Tb(OH)3. In solution, it forms trivalent species but may be oxidized to the tetravalent state with ozone gas in a highly basic medium.
2 Tb + 6 H2O → 2 Tb(OH)3 + 3 H2
The rare earth metal Tb is a strong reducing agent that reduces various metal oxides to form their elemental state. The metal Tb is readily attacked by dilute sulfuric acid to form a colorless solution of Tb (III) ions.
2 Tb + 3 H2SO4 → 2 Tb3+ + 3 SO4−2 + 3 H2
Terbium Interesting Facts
- It is a soft, malleable, ductile, silver-gray rare earth metal of the lanthanide family of the periodic table.
- Terbium is one of the rarer rare-earth metals that is more common than the noble metal silver.
- It is a yttrium group of lanthanide or heavier rare earth elements.
- The green phosphors of terbium are found in color TV tubes, trichromatic lighting, and fluorescent lamps.
- It is a mildly toxic rare earth metal that has no known biological functions.
- Terbium powder and compounds are very irritating when they come into contact with our skin and eyes.
Uses of Terbium
- It is used in solid-state devices as a stabilizer of fuel cells by dopping terbium with calcium fluoride, calcium tungstate, and strontium molybdate.
- It is alloyed with dysprosium and iron to maintain the strength of the magnetic field. Such technology is used in actuators, naval sonar systems, sensors, SoundBug devices, and other magneto-mechanical devices.
- It may be used to improve the safety of medical x-rays devices by allowing the same quality images.
- The green phosphors terbium oxide is used for making color tv tubes, monitor cathode-ray tubes, low-energy lightbulbs, and mercury lamps.
- The isotope 160Tb is used to study co-precipitation and chromatographic separation.
- Various terbium salts are used for the manufacture of laser devices.
- The green phosphorus of terbium is used for making trichromatic lighting technology when combine with divalent europium blue phosphors and trivalent europium red phosphors. Trichromatic lighting technology provides much higher light output for a given amount of electrical energy.